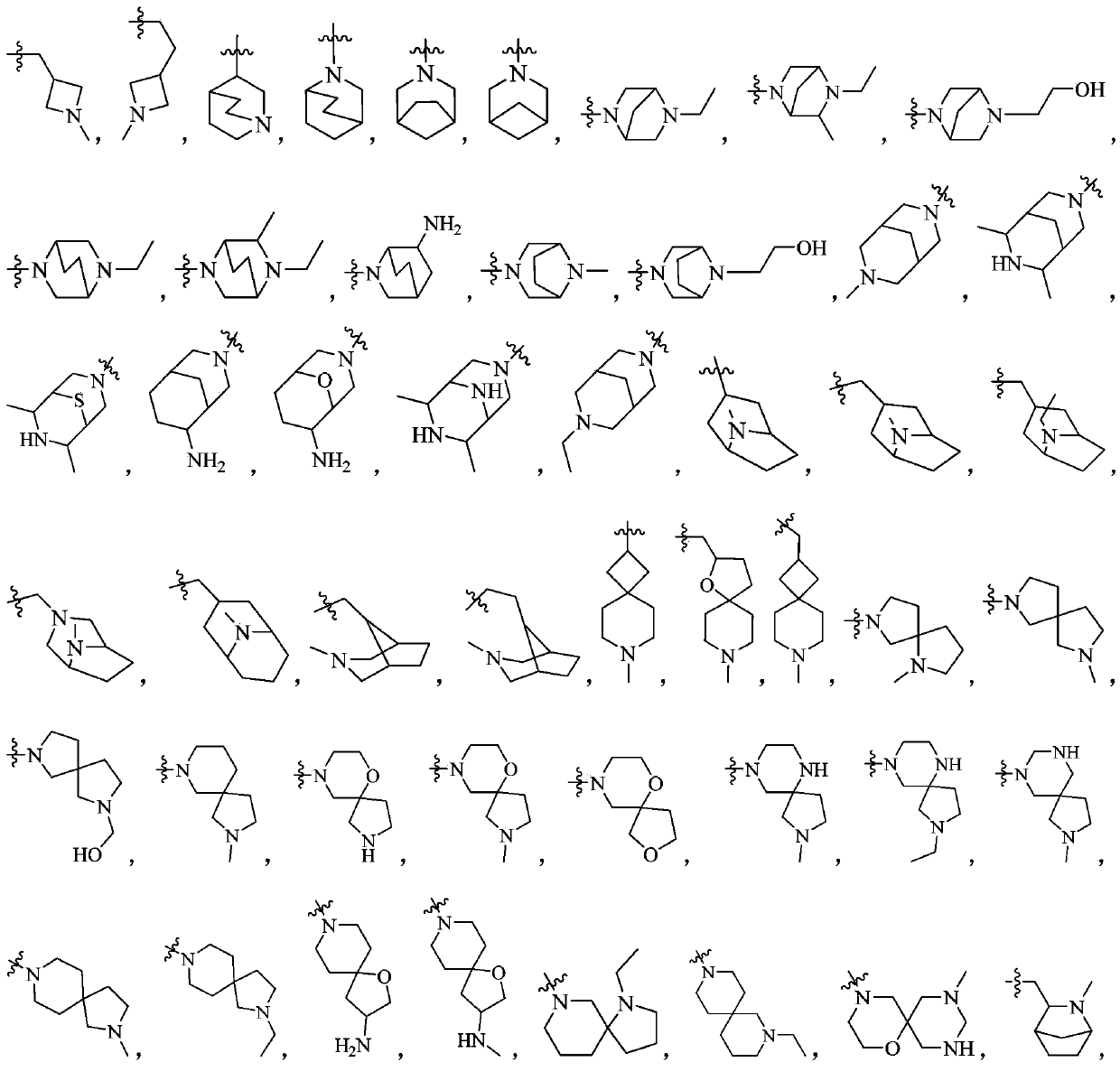
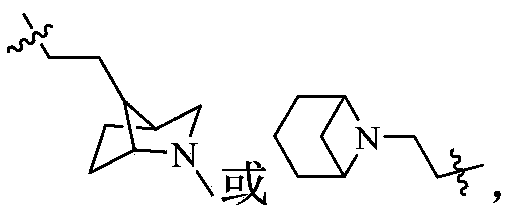
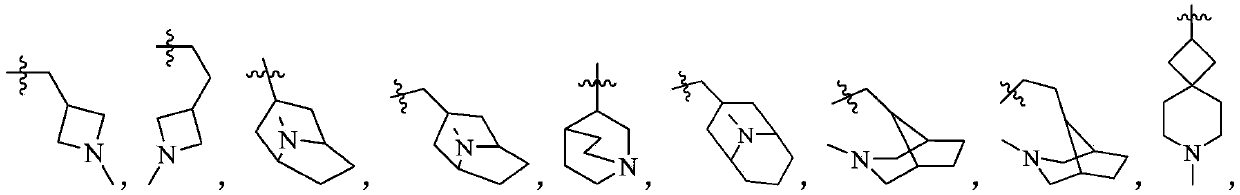
Phosphodiesterase-5 inhibitor
A compound, hydroxyl technology, applied in the field of medicine, can solve the problems of short half-life and inability to treat diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0150] Example 14-ethoxy-3-(5-methyl-4-oxo-7-propyl-3,4-dihydroimidazol[5,1-f][1,2,4]triazine- 2- Base)-N-(((1R,5S)-9-methyl-9-azabicyclo[3.3.1]nonan-3-yl)methyl)benzenesulfonamide (compound 1)
[0151]
[0152] (1) Preparation of (1R,5S)-9-(4-methoxybenzyl)-9-azabicyclo[3.3.1]nonan-3-one (intermediate 1-2)
[0153] Add 3-oxoglutaric acid (raw material 1-1) (5.0g, 0.034mol) and p-methoxybenzylamine (20mL) into a 250mL eggplant-shaped bottle, under nitrogen protection, heat up to 80°C, and stir for 10h . Dichloromethane was added, stirred and filtered with suction, and the filtrate was distilled and purified by column chromatography to obtain 5.0 g of light red oily substance of Intermediate 1-2, with a yield of 56%.
[0154] (2) Preparation of (1R,5S)-9-(4-methoxybenzyl)-9-azabicyclo[3.3.1]nonane-3-carbonitrile (intermediate 1-3)
[0155] Add intermediate 1-2 (5.1g, 19.69mmol), TOSmic (p-toluenesulfonyl isonitrile, 6.91g, 35.44mmol) and ethylene glycol dimethyl ether ...
Embodiment 24
[0168] Example 24-ethoxy-N-(2-((1S,8R)-3-methyl-3-azabicyclo[3.2.1]octane-8-yl)ethyl)-3-(5 -Methyl-4- Preparation of oxo-7-propyl-3,4-dihydroimidazo[5,1-f][1,2,4]triazin-2-yl)benzenesulfonamide (compound 2)
[0169]
[0170] (1) Preparation of 2-((1R,5S)-3-azabicyclo[3.2.1]octane-8-yl)acetic acid (intermediate 2-2)
[0171] 2-((1R,5S)-3-(tert-butoxycarbonyl)-3-azabicyclo[3.2.1]octane-8-yl)acetic acid (raw material 2-1) (0.5g, 1.86mmol) Dissolve in 10mL CH 2 Cl 2 , adding 5mL of TFA, stirring at room temperature for 3 hours, the reaction solution was rotary evaporated to dryness under reduced pressure to obtain intermediate 2-2 (0.28g), the yield was 89%.
[0172] (2) Preparation of 2-((1R,5S)-3-methyl-3-azabicyclo[3.2.1]octane-8-yl)acetic acid (intermediate 2-3)
[0173] Intermediate 2-2 (0.25 g, 1.48 mmol) was dissolved in 10 mL of methanol at room temperature, and aqueous formaldehyde (37%) was added dropwise, and the reaction was stirred overnight under 1 atm of ...
Embodiment 34
[0188] Example 34-ethoxy-N-(((1R,5S)-3-methyl-3-azabicyclo[3.2.1]octane-8-yl)methyl)-3-(5-methyl yl-4-oxo Preparation of substituted-7-propyl-3,4-dihydroimidazo[5,1-f][1,2,4]triazin-2-yl)benzenesulfonamide (Compound 3)
[0189]
[0190] (1) Preparation of (1R,5S,8s)-3-azabicyclo[3.2.1]octane-8-carboxylic acid (intermediate 3-2)
[0191] Dissolve (1R,5S,8s)-3-(tert-butoxycarbonyl)-3-azabicyclo[3.2.1]octane-8-carboxylic acid (raw material 3-1) (0.5g, 2.0mmol) in 10mL CH 2 Cl 2 5 mL of TFA was added, and after stirring at room temperature for 3 hours, the reaction solution was spin-dried to obtain intermediate 3-2 (0.26 g), with a yield of 84%.
[0192] (2) Preparation of (1R,5S,8s)-3-methyl-3-azabicyclo[3.2.1]octane-8-carboxylic acid (intermediate 3-3)
[0193] At room temperature, the intermediate 3-2 (0.26g, 1.7mmol) was dissolved in 10mL of methanol, 0.2mL of formaldehyde aqueous solution (37%) was added dropwise, and stirred overnight under 1 atm of hydrogen gas. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com