Detection kit and detection method for 19 deletion mutations of EGFR gene exon 19
A detection kit and deletion mutation technology, which is applied in the fields of biotechnology and medicine, can solve problems such as unsatisfactory detection results, and achieve simple and objective result interpretation, high detection sensitivity, and simple and effective detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Preparation of the EGFR gene exon 19 mutation detection kit specifically includes the following steps:
[0054] 1. Synthetic primers and probe sequences
[0055] Synthetic primer sequence SEQ ID NO:1, SEQ ID NO:2; Synthetic specific probe sequence SEQ ID NO:3, and labeled with FAM fluorescent group at the 5' end, and labeled with quenching groups NFQ and MGB at the 3' end modifying group.
[0056] The above primer sequences were respectively prepared into 100 μM mother solution for storage, and the above probe sequences were respectively prepared into 100 μM mother solution for storage.
[0057] 2. Synthetic Blocking Nucleic Acid Sequence
[0058] Synthetic blocking nucleic acid sequence SEQ ID NO:4, and a phosphate group (PO) at the 3' end 4 ) modification.
[0059] The blocking nucleic acid was prepared as a 100 μM stock solution for storage.
[0060] 3. Preparation of fluorescent quantitative PCR reaction system
[0061] Prepare the quality control detection re...
Embodiment 2
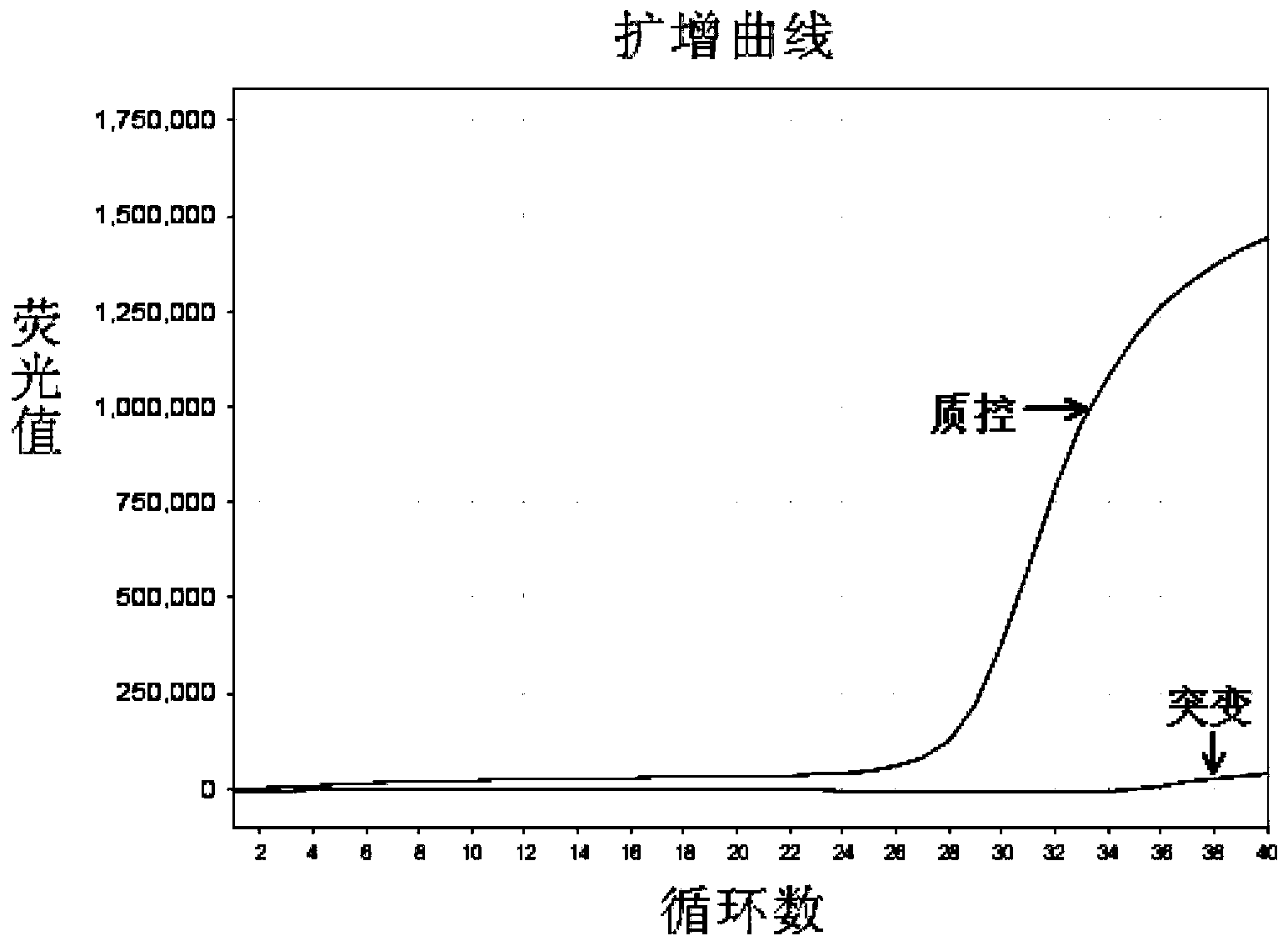
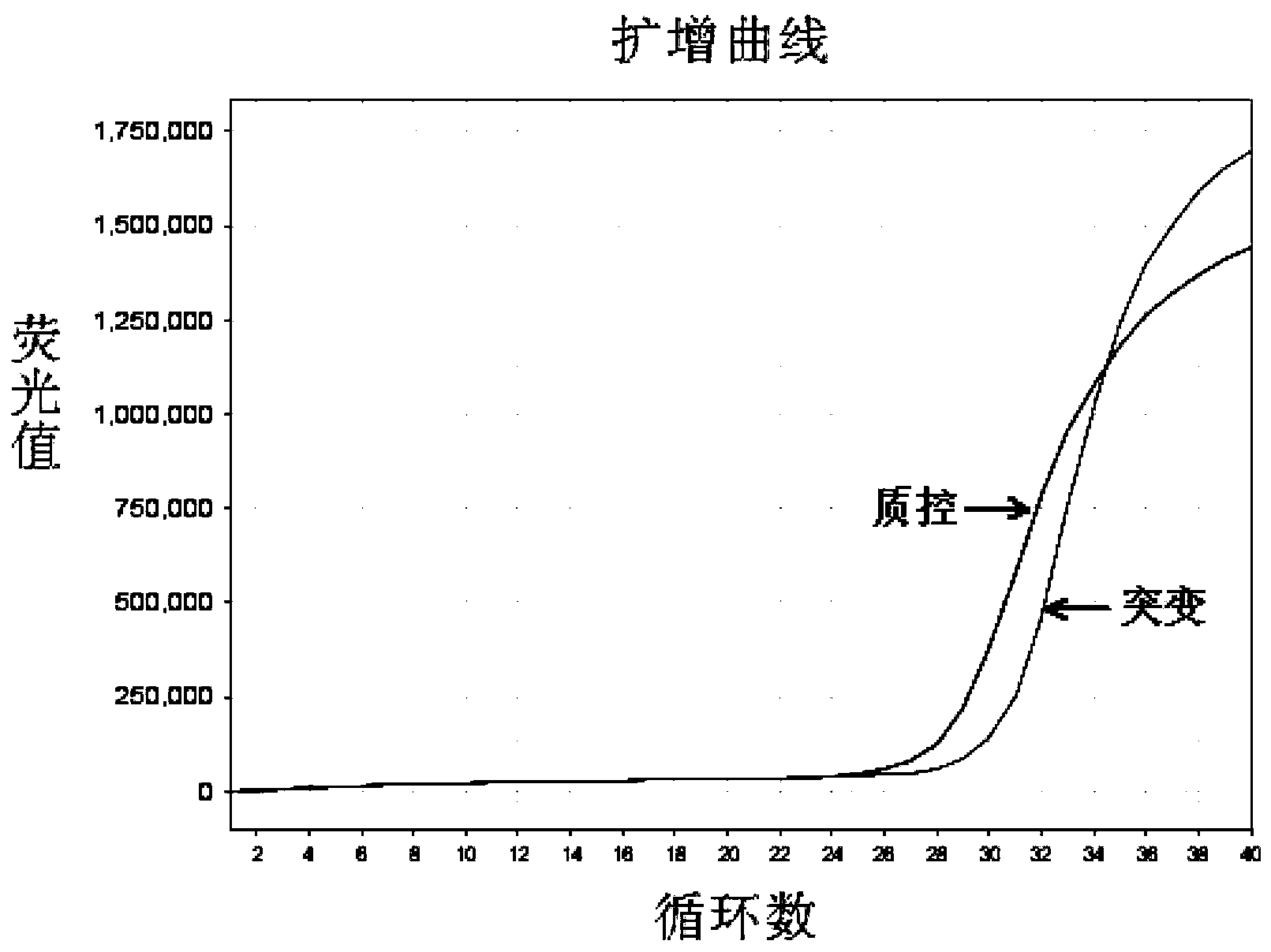
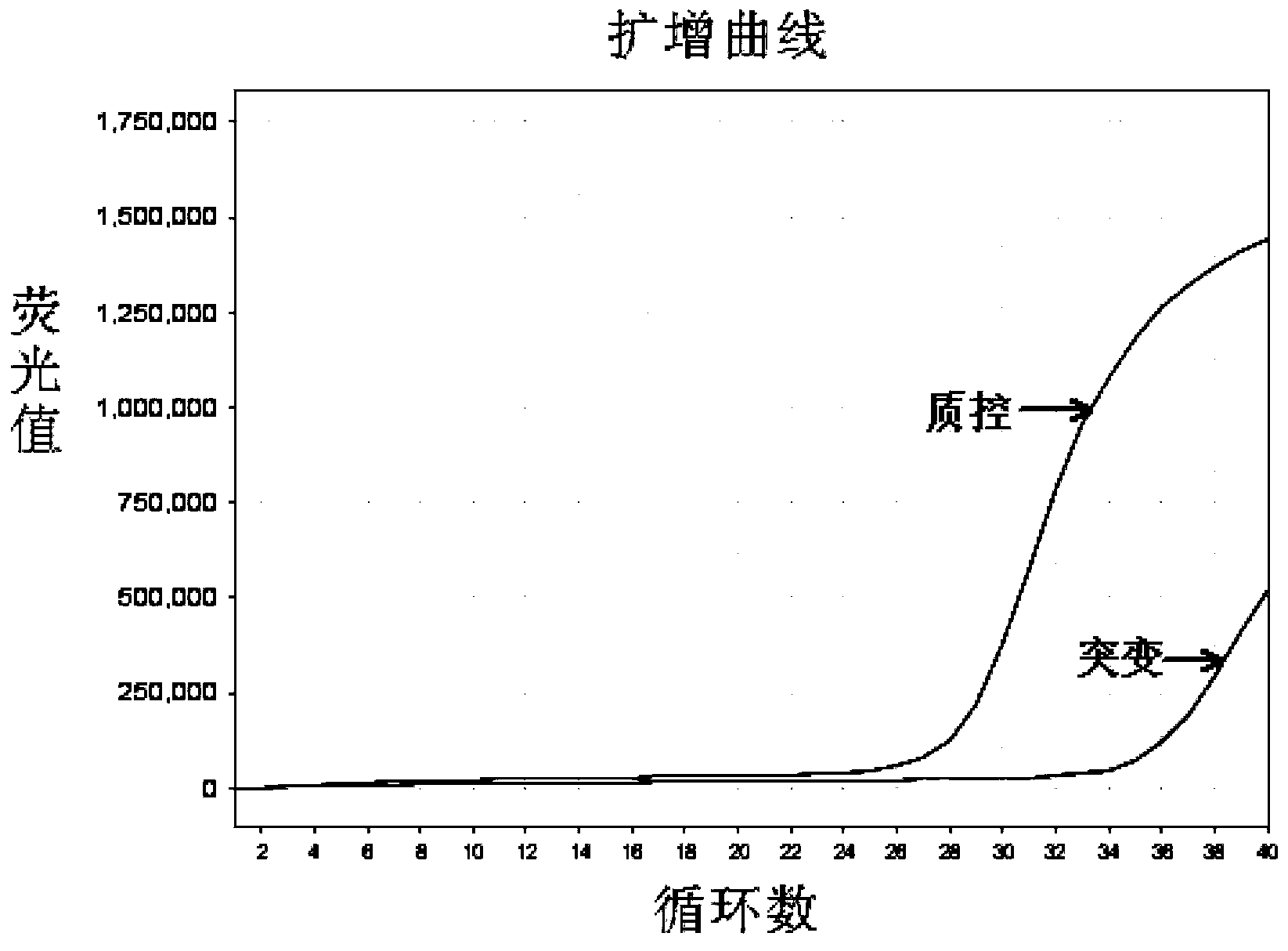
[0067] The EGFR gene exon 19 mutation detection kit prepared in Example 1 was used to detect the samples to be tested.
[0068] In this embodiment, 41 cases of paraffin-embedded tissue sections of patients with clinical and pathological diagnosis of non-small cell lung cancer were collected, and genomic DNA was extracted therefrom, and the samples to be tested were detected with the EGFR gene exon 19 mutation detection kit obtained in Example 1 Whether there is a deletion mutation of exon 19 of the EGFR gene in the test, and the traditional sequencing method is used to verify. The specific operation steps are:
[0069] 1. Extraction of Genomic DNA from Samples
[0070]Genomic DNA was extracted from the tissue samples of the lung cancer patients above using a DNA extraction kit (QIAamp DNA FFPE Tissue Kit, Cat No. 56404). The DNA extraction method is carried out according to the instructions, and the operation steps are briefly described as follows: Place the clinically collec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com