Preparation process for thiamine hydrochloride
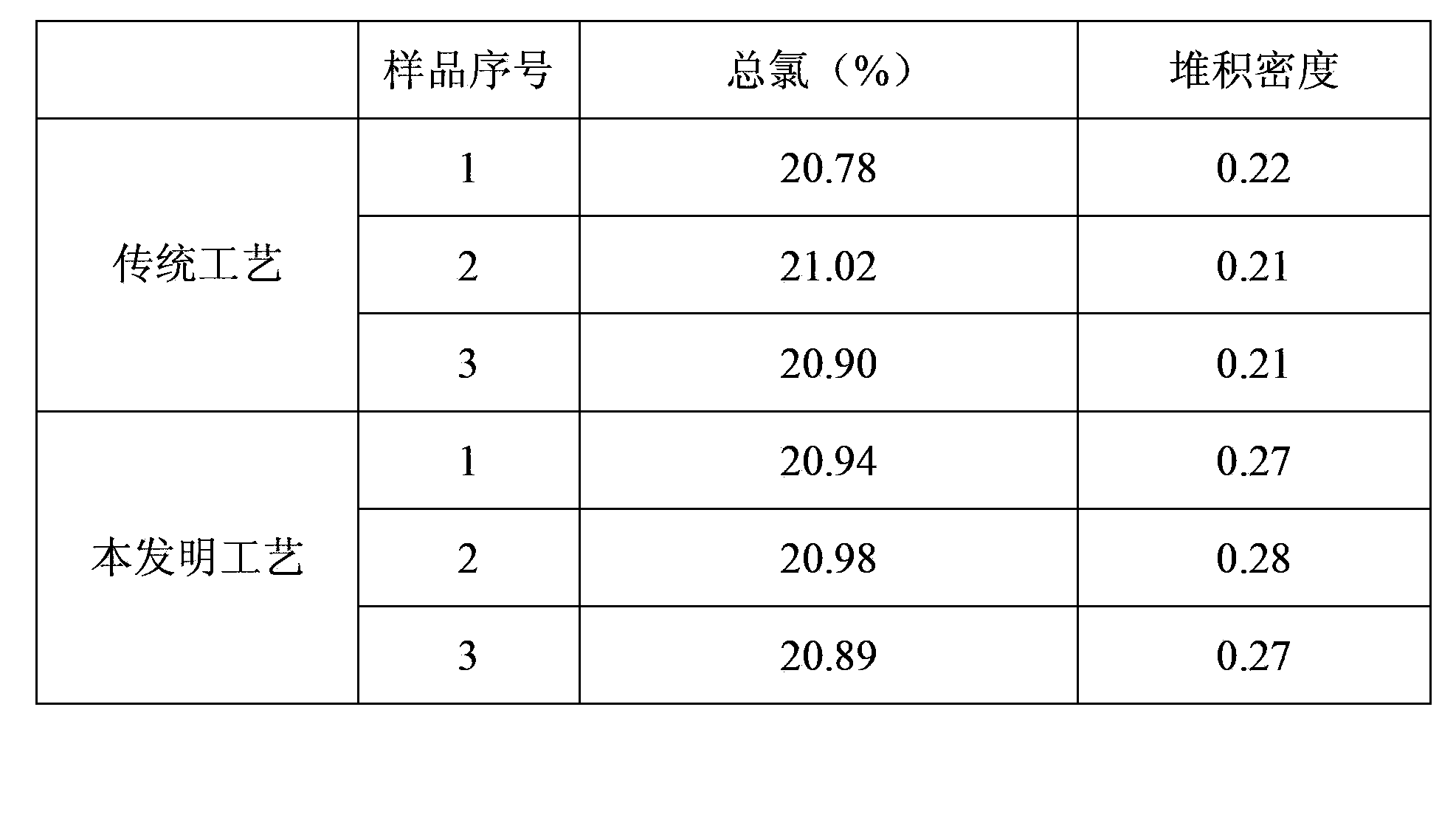
A technology for the preparation of thiamine hydrochloride, which is applied in the direction of organic chemistry, can solve the problems of low bulk density, poor fluidity, and large fluctuations in total chlorine content of thiamine hydrochloride, so as to reduce the risk of environmental pollution and solve the fluctuation of total chlorine Larger and more stable total chlorine content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) Preparation of hydrogen chloride gas:
[0018] Weigh 700.5g of 31% hydrochloric acid and heat it to 110°C. The volatilized hydrogen chloride gas is condensed by a condenser tube and then scrubbed with concentrated sulfuric acid to dry and dehydrate.
[0019] (2) Preparation of acid methanol solution:
[0020] Pass the dried hydrogen chloride gas into 88g of methanol for absorption. After three hours, detect the mass concentration of hydrogen chloride in the prepared acid methanol, and stop the ventilation when it reaches 33%. The entire preparation process takes about 3.5 hours.
[0021] (3) Preparation of thiamine hydrochloride:
[0022] Add 100.1 g of thiamine nitrate and 500.0 g of methanol into a four-neck flask to prepare a methanol solution of thiamine nitrate; control the temperature at 0-70°C, and add the prepared methanol hydrochloric acid solution dropwise to the methanol of thiamine nitrate In the solution, it took 2 hours and 10 minutes. After the drop...
Embodiment 2
[0024] (1) Preparation of hydrogen chloride gas:
[0025] Weigh 700.2g of 31% hydrochloric acid and heat it to 110°C. The volatilized hydrogen chloride gas is condensed by a condenser tube and then scrubbed with concentrated sulfuric acid to dry and dehydrate.
[0026] (2) Preparation of acid methanol solution:
[0027] Pass the dried hydrogen chloride gas into 88g of methanol for absorption. After three hours, detect the mass concentration of hydrogen chloride in the prepared acid methanol, and stop the ventilation when it reaches 33%. The entire preparation process takes about 3 hours and 20 minutes.
[0028] (3) Preparation of thiamine hydrochloride:
[0029] Add 100.0 g of measured thiamine nitrate and 500.5 g of methanol into a four-neck flask to prepare a methanol solution of thiamine nitrate; control the temperature at 0-70°C, and add dropwise the prepared methanol hydrochloric acid solution to the methanol of thiamine nitrate In the solution, it took 2 hours and 05 m...
Embodiment 3
[0031] (1) Preparation of hydrogen chloride gas:
[0032] Weigh 700.3g of 31% hydrochloric acid and heat it to 110°C. The volatilized hydrogen chloride gas is condensed by a condenser tube and then scrubbed with concentrated sulfuric acid to dry and dehydrate.
[0033] (2) Preparation of acid methanol solution:
[0034] Pass the dried hydrogen chloride gas into 88g of methanol for absorption. After three hours, detect the mass concentration of hydrogen chloride in the prepared acid methanol, and stop the ventilation when it reaches 33%. The entire preparation process takes about 3 hours and 30 minutes.
[0035] (3) Preparation of thiamine hydrochloride:
[0036] Add 100.1 g of measured thiamine nitrate and 500.4 g of methanol into a four-neck flask to prepare a methanol solution of thiamine nitrate; control the temperature at 0-70°C, and add the prepared methanol solution of hydrochloric acid dropwise to the methanol of thiamine nitrate In the solution, it took 2 hours and 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com