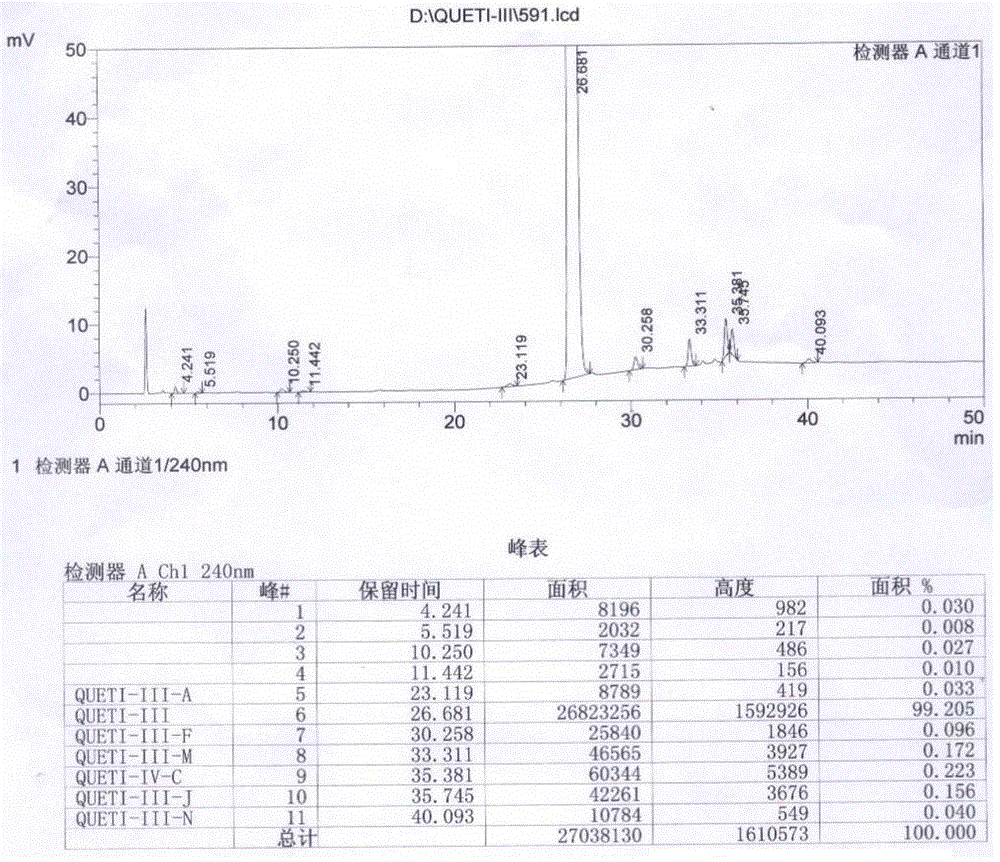
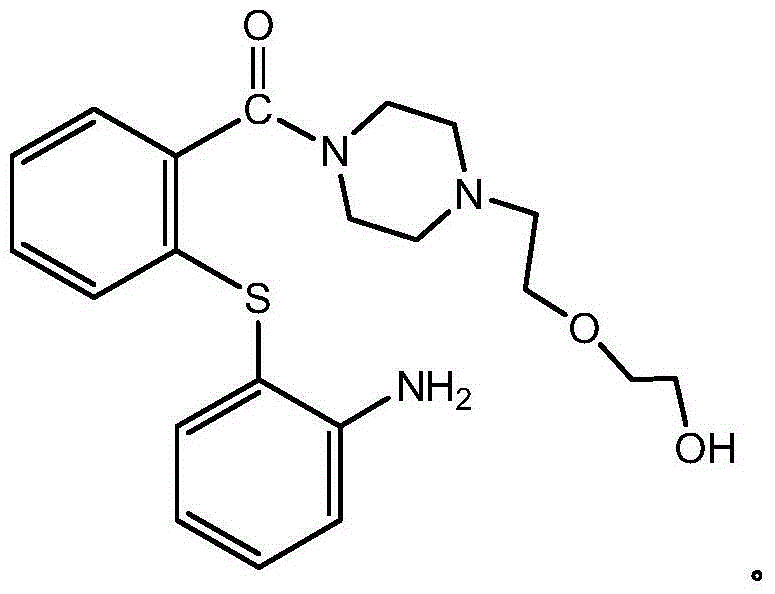
Preparation method of quetiapine intermediate
A technology of equations and reactions, applied in the direction of organic chemistry, etc., to achieve the effects of low cost, mild reaction conditions, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The present invention will be described in further detail below in conjunction with specific embodiments.
[0028] A kind of [2-(2-amino-phenylmercapto)-phenyl]-{4-[2-(2-hydroxy-ethoxy)-ethyl]-piperazin-1-yl}-methanone hydrochloride The preparation method of salt (hereinafter referred to as Queti-III), it comprises the steps:
[0029] (1), preparation of 2-iodobenzoyl chloride
[0030]
[0031] Add 600g of toluene, 390g of 2-iodobenzoic acid and 380g of thionyl chloride into a 2000ml four-neck flask, heat the mixture for about 1-2 hours to boiling (68-75°C) and stir rapidly, a large amount of HCl and SO2 will be produced during heating . Heat to 90°C over 2-3 hours, stirring rapidly. Stir rapidly at 90°C for at least 6 hours. After the heat preservation, the reaction solution was cooled to 35±10°C. Evaporate excess SOCl2 and toluene under reduced pressure, under rapid stirring, until the final temperature is 90°C, and the final vacuum degree is less than -0.09MP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com