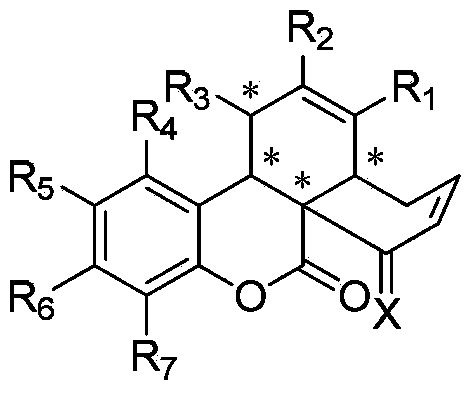
Coumarin skeleton polycyclic compound with biological activity as well as preparation method and use thereof
A technology of coumarin skeleton and polycyclic compounds, which is applied in the field of medicinal chemistry, can solve problems such as no relevant reports, and achieve the effects of good atom economy, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
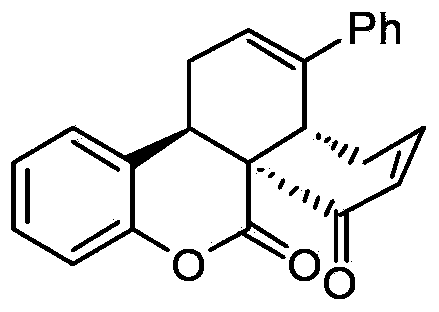
[0035] In a clean 10ml reaction tube, add chiral prolinol silyl ether catalyst (0.02mmol, 14.8mg), 3-acetylcoumarin (0.1mmol, 18.8mg), 4-phenyl-2,4 -Hexadienal (0.2mmol, 34mg), L-Boc-2-phenylglycine (0.02mmol, 5mg) and chloroform (0.5mL), stirred and reacted at 0°C for 42 hours, after the reaction was monitored by TLC, added DBU (0.05mmol, 7.5μL), stirred at 25°C for 2 hours, then added TEA (0.5mmol, 70μL) and MsCl (0.2mmol, 15.5μL) at 0°C and stirred for 15 minutes, recovered the solvent under reduced pressure, and the residue was passed through The product P1 was separated by column chromatography and named as (3aR,7aS,13bR)-3-phenyl-1,3a,4,13b-tetrahydronaphtho[8a,1-c]benzopyran-7,8- Diketone, yield rate is 87%, structural formula is as follows:
[0036]
[0037] [α] D 20 =+152.4(c=0.42in CHCl 3 ); 90%ee, chiral test conditions HPLC analysis [Daicel chiralpak AD, n-hexane / i-PrOH=80 / 20, 1.0mL / min, λ=254nm, t(major)=18.53min, t(minor) =11.84min];
[0038] 1 H NMR (4...
Embodiment 2
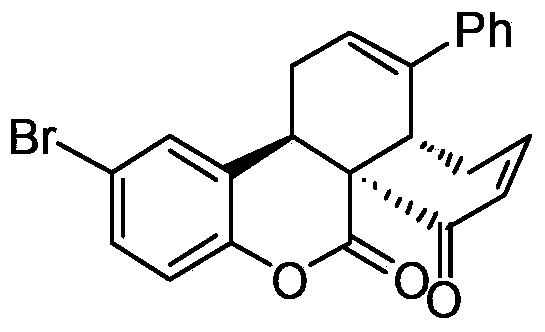
[0042] In a clean 10ml reaction tube, add chiral prolinol silyl ether catalyst (0.02mmol, 14.8mg), 6-bromo-3-acetylcoumarin (0.1mmol, 26.7mg), 4-phenyl -2,4-Hexadienal (0.2mmol, 34mg), L-Boc-2-phenylglycine (0.02mmol, 5mg) and chloroform (0.5mL), stirred the reaction at 0°C for 72 hours, and monitored the reaction by TLC After the end, add DBU (0.05mmol, 7.5μL), stir at 25°C for 2 hours, then add TEA (0.5mmol, 70μL) and MsCl (0.2mmol, 15.5μL) at 0°C and stir for 15 minutes, then recover the solvent under reduced pressure , the residue was separated by column chromatography to obtain the product P2 name: (3aR,7aS,13bR)-12-bromo-3-phenyl-1,3a,4,13b-tetrahydronaphtho[8a,1-c] The yield of benzopyran-7,8-dione is 76%. The structural formula is as follows:
[0043]
[0044] [α] D 20 =+162.9(c=0.80in CHCl 3 );87%ee, chiral test conditions HPLC analysis[Daicel chiralpak AD,n-hexane / i-PrOH=80 / 20,1.0mL / min,λ=254nm,t(major)=19.07min,t(minor) =17.72min];
[0045] 1 H NMR (400MHz...
Embodiment 3
[0049] In a clean 10ml reaction tube, add chiral prolinol silyl ether catalyst (0.02mmol, 14.8mg), 6-chloro-3-acetylcoumarin (0.1mmol, 22.2mg), 4-phenyl -2,4-Hexadienal (0.2mmol, 34mg), L-Boc-2-phenylglycine (0.02mmol, 5mg) and chloroform (0.5mL), stirred the reaction at 0°C for 12 hours, and monitored the reaction by TLC After the end, add DBU (0.05mmol, 7.5μL), stir at 25°C for 2 hours, then add TEA (0.5mmol, 70μL) and MsCl (0.2mmol, 15.5μL) at 0°C and stir for 15 minutes, then recover the solvent under reduced pressure , the residue was separated by column chromatography to obtain the product P3 name: (3aR,7aS,13bR)-12-chloro-3-phenyl-1,3a,4,13b-tetrahydronaphtho[8a,1-c] The yield of benzopyran-7,8-dione is 83%. The structural formula is as follows:
[0050]
[0051] [α] D 20 =+204.4(c=0.78in CHCl 3 );87%ee, chiral test conditions HPLC analysis[Daicel chiralpak AD,n-hexane / i-PrOH=80 / 20,1.0mL / min,λ=254nm,t(major)=20.04min,t(minor) =18.11min];
[0052] 1 H NMR (400M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com