Topiramate pharmaceutical composition
A composition and a technology for topiramate, which are applied in the field of topiramate pharmaceutical compositions and their preparation, can solve the problems that tablets are prone to splitting, the operation process is cumbersome, the preparation method is cumbersome and the like, and the quality and safety are guaranteed, and the process is simple and convenient. Effects of storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Topiramate 20mg
[0035] Pregelatinized Starch 42mg
[0036] Sodium carboxymethyl starch 1.2mg
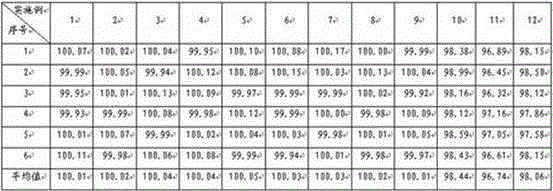
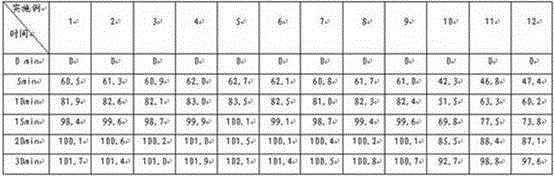
[0037]Preparation method: Weigh topiramate, pregelatinized starch, and sodium carboxymethyl starch in the prescribed amount, pulverize them separately, sieve, and mix evenly to prepare a mixed powder, use a dry granulator to make the mixed powder into large pieces, pulverize, and collect 250 μm Granules between -550μm and drug-containing granules are prepared, and the rest of the materials continue to dry granulate until the proportion of collected particles between 250μm and 550μm exceeds 75%, and they are compressed into tablets or loaded into capsules.
Embodiment 2
[0039] Topiramate 28mg
[0040] Pregelatinized Starch 56mg
[0041] Low-substituted hydroxypropyl cellulose 2.7mg
[0042] Prepare according to the method in Example 1.
Embodiment 3
[0044] Topiramate 30mg
[0045] Pregelatinized starch 60mg
[0046] Sodium carboxymethyl starch 2.0mg
[0047] Low-substituted hydroxypropyl cellulose 1.0mg
[0048] Prepared according to the method in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com