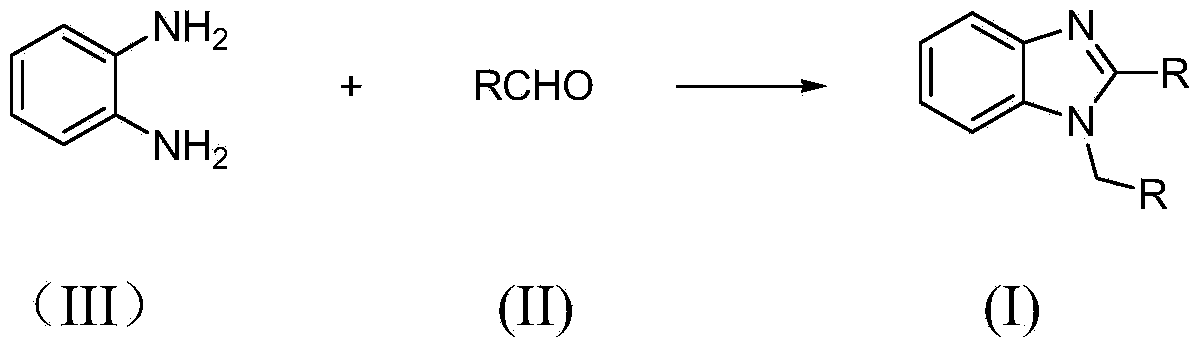
1,2-disubstituted benzimidazole compound preparation method
A technology of benzimidazole and aldehyde compounds, which is applied in the field of preparation of benzimidazole compounds, can solve the problems of unsuitable recovery of catalysts, limited scope of application, increase of dangerous types, etc., and achieve high raw material utilization, convenient operation, and reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
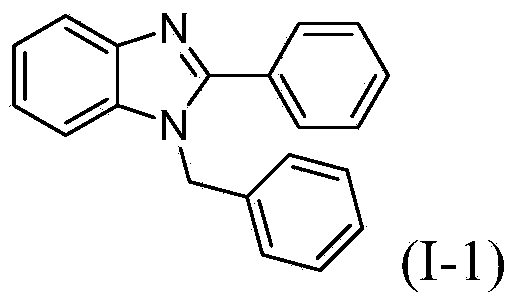
[0021] Example 1: Compound (I-1)
[0022] O-phenylenediamine (0.1080g, 1mmol), benzaldehyde (233.5ul, 2.2mmol), nano-zinc oxide (purchased from Aladdin Reagent Company, particle size 30±10nm) (0.0082g, 0.1mmol) in 1, Mixed with 1,2,2-tetrachloroethane (2 mL), stirred in an oil bath at 60°C for reaction, followed by TLC, and the reaction was complete in 1 hour. After the reaction, extract with ethyl acetate (10mL×3), wash with saturated brine, combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate, the concentrate is separated by column chromatography, and the volume of petroleum ether and ethyl acetate Ratio = 8:1 mixed solvent as eluent, collect R f The eluate with a value of 0.3-0.35 was evaporated under reduced pressure to remove the solvent, and dried to obtain 0.2095 g of the target compound (I-1), with a yield of 73.7%.
[0023]
[0024] 1 H NMR (500MHz, CDCl 3 ):δ7.91(d,J=7.9Hz,1H),7.76–7.67(m,2H),7.51–7.46(m,3H),7.38–7.31(m,4H),7.27–7...
Embodiment 2
[0026] Change the solvent to CHCl 3 (0.5ml), other operation is with embodiment 1, and yield is 74.4%.
Embodiment 3
[0028] The solvent was replaced with water, the reaction time was 1.5h, other operations were the same as in Example 1, and the yield was 67.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com