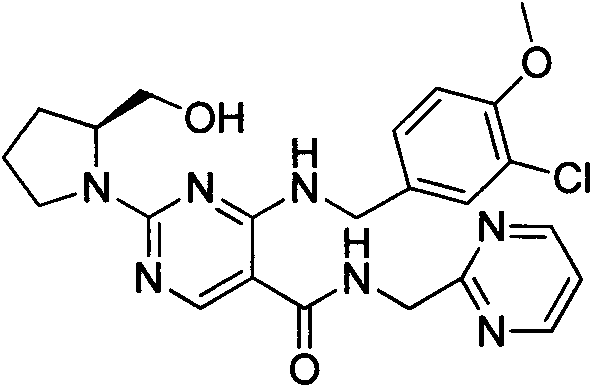
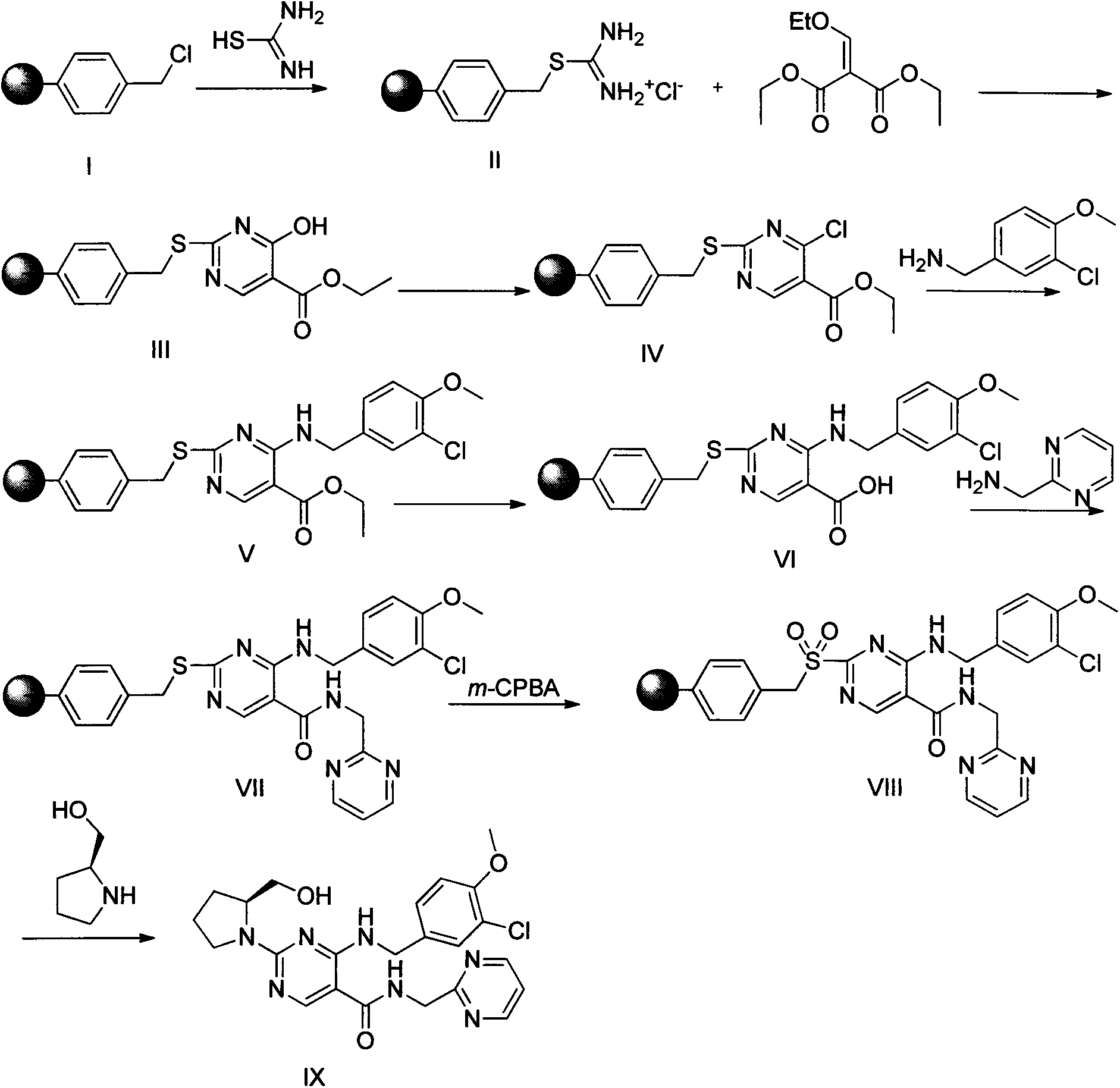
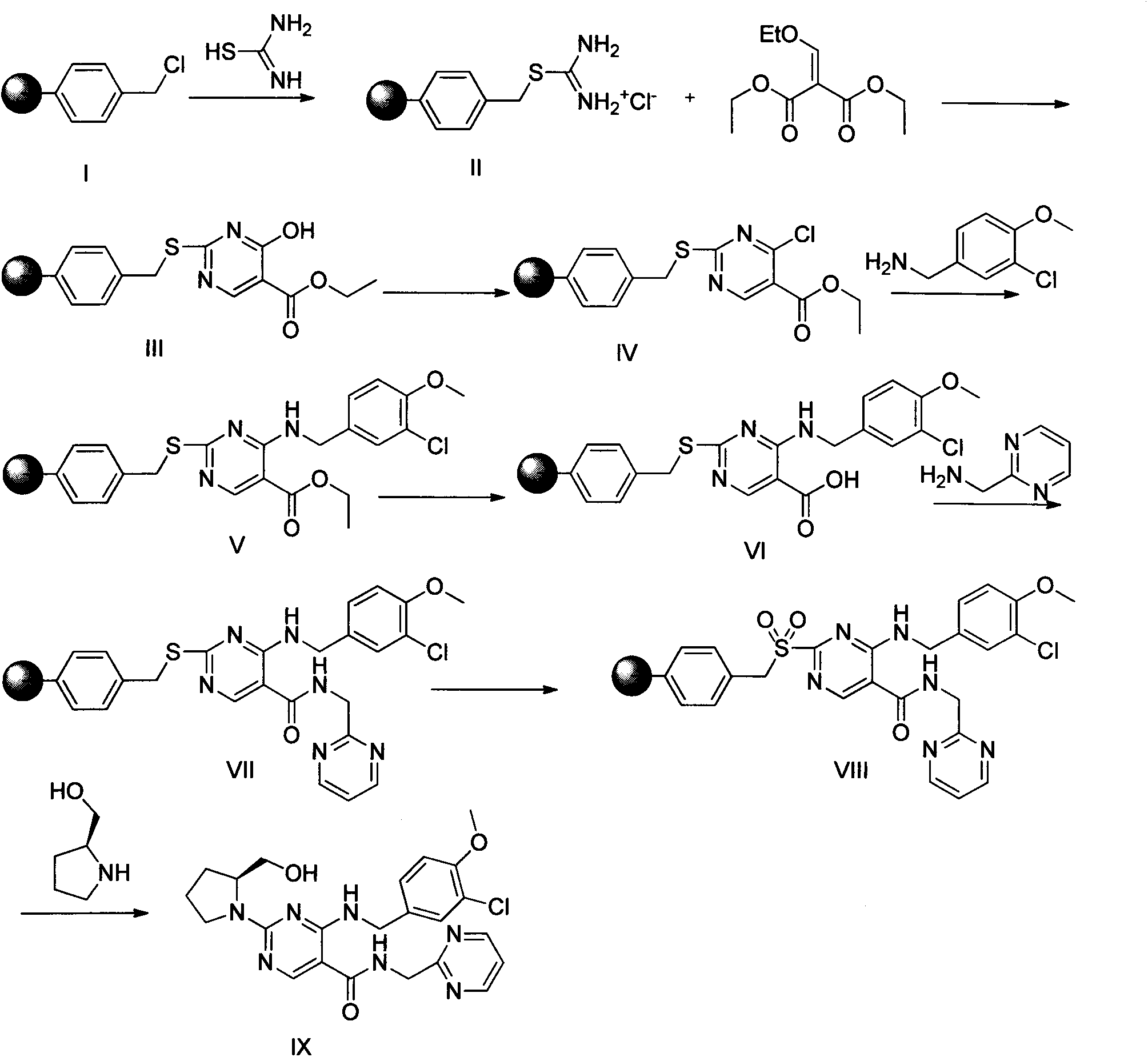
Solid-phase preparation method of avanafil
A solid-phase preparation, Avanafil technology, applied in the direction of organic chemistry, etc., to achieve the effect of easy post-processing, good application prospects, and easy to achieve purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the synthesis of compound (II):
[0047] The high load Merrifiled resin (2.15mmol / g, 5g) was put into the reactor, and thiourea (4g, 53.75mmol) was dissolved in 200mL of dioxane-ethanol solution with a volume ratio of 4:1, and added into the reactor, stirred at 85°C for 15h, washed four times with ethanol at 70°C, washed twice each with dioxane and pentane at room temperature, and dried the obtained solid in vacuum at 60°C to obtain compound (II )6g.
Embodiment 2
[0048] Embodiment 2: the synthesis of compound (III):
[0049] The product (II) (3 g, 5.375 mmol) was put into a solid phase reactor, 10 mL of DMF was added, shaken at room temperature for 5 min, and N,N-diisopropylethylamine (2.3 mL, 13.438 mmol) was added slowly. Then diethyl ethoxymethylene malonate (5.8 g, 26.875 mmol) was dissolved in 5 mL of DMF, slowly added into the reactor, and shaken overnight at room temperature. After the reaction was completed, filter and wash with DMF, ethanol, and dichloromethane in sequence, and the obtained solid was vacuum-dried at 60° C. to obtain compound (III).
Embodiment 3
[0050] Embodiment 3: the synthesis of compound (IV):
[0051] POCl 3 (20mL, 21.8mmol) was cooled to 10°C and added to the reactor, and compound (III) (2.82g, 4.36mmol) was added to the reactor in batches, keeping the temperature not exceeding 25°C, and then heated to 65-80 ℃ for 3 to 5 hours, after the reaction is completed, filter off excess POCl 3 , the obtained solid was washed successively with ice water, ethanol and dichloromethane, and dried under vacuum at 60° C. to obtain compound (IV).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com