Rapamycin-induced regulatory γδt cell culture method
A technology of rapamycin and a culture method, which is applied in the field of immunology research to achieve the effects of strong feasibility, strong immunosuppressive function, and good promotion value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of mononuclear cells derived from human peripheral blood
[0021] 1. Dilute the collected heparin anticoagulated peripheral blood (Zhejiang Blood Center) with the same amount of PBS (phosphate buffered saline) at 1:1;
[0022] 2. Take several 15ml centrifuge tubes, add 5ml of human lymphocyte separation solution to each, and slowly superimpose the diluted peripheral blood on the lymphocyte separation solution along the tube wall. Add 10ml of peripheral blood to each centrifuge tube, that is, 5ml lymph. Cell separation solution + 10ml diluted peripheral blood (1:2); horizontal centrifugation, 500g×20 minutes, room temperature;
[0023] 3. After centrifugation, the tube is divided into three layers, the upper layer is plasma and PBS buffer, the lower layer is mainly red blood cells and granulocytes, the middle layer is lymphocyte separation liquid, and there is a white layer dominated by mononuclear cells at the interface of the upper and middle layers. T...
Embodiment 2
[0026] Example 2 Induction and culture of regulatory γδT cells
[0027] 1. Divide the cultured cells into three major groups, including the IL-2 culture group alone (γδT group), the TGF-β1 / IL-2 / IL-15 induction group (TGF-β1 group), and the combined TGF- β1 / IL-2 / IL-15 and rapamycin induction group (combined with rapamycin group); each cytokine was added on the 0th day of cell culture, and the final concentration after addition is shown in Table 1 below; Rapamycin was also added on day 0 of cell culture.
[0028]
[0029] 2. On the 3rd, 6th, and 9th days, perform a half-volume exchange and add each corresponding cytokine to keep the final concentration of each cytokine consistent.
[0030] 3. The storage concentration of each cytokine are: Zoledronic acid (56mmol / ml), IL-2 (2×10 4 U / ml), IL-15 (10μg / ml), TGF-β1 (1μg / ml), rapamycin (10μM); store at -20°C.
Embodiment 3
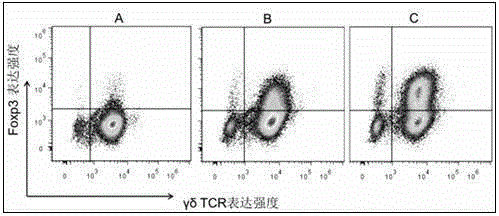
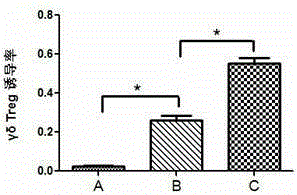
[0031] Example 3 Analysis and comparison of the induction of regulatory γδ T cells under different culture conditions
[0032] At present, the in vitro induction of regulatory γδ T cells is more commonly used by the method of IL-2 / IL-15 / TGF-β1 induction. This experiment is based on IL-2 / IL-15 / TGF-β1 and further combined applications Rapamycin is synergistically induced, and the changes in its induction efficiency are tested and compared.
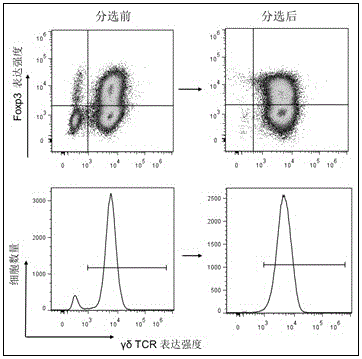
[0033] 1. Collect cells cultured for 15 days under different induction culture conditions, that is, only the IL-2 culture group (γδT group, as a control), and the TGF-β1 / IL-2 / IL-15 culture group (TGF-β1 group) ) And the combined application of rapamycin and TGF-β1 / IL-2 / IL-15 culture group (combined rapamycin group), flow cytometry detection and analysis of the content of regulatory γδT cells.
[0034] 2. The method for detecting the content of regulatory γδT cells by flow cytometry is as follows:
[0035] (1) The harvested cells are placed in a fl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com