Tablet capsule filled with topiramate tablets
A technology of topiramate tablets and topiramate tablets, applied in the direction of medical preparations containing active ingredients, organic active ingredients, drug delivery, etc., can solve the problems of reducing patient compliance, trouble, and inconvenience, and reduce the trouble of taking medicine , improve the effect of treatment, increase the effect of compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
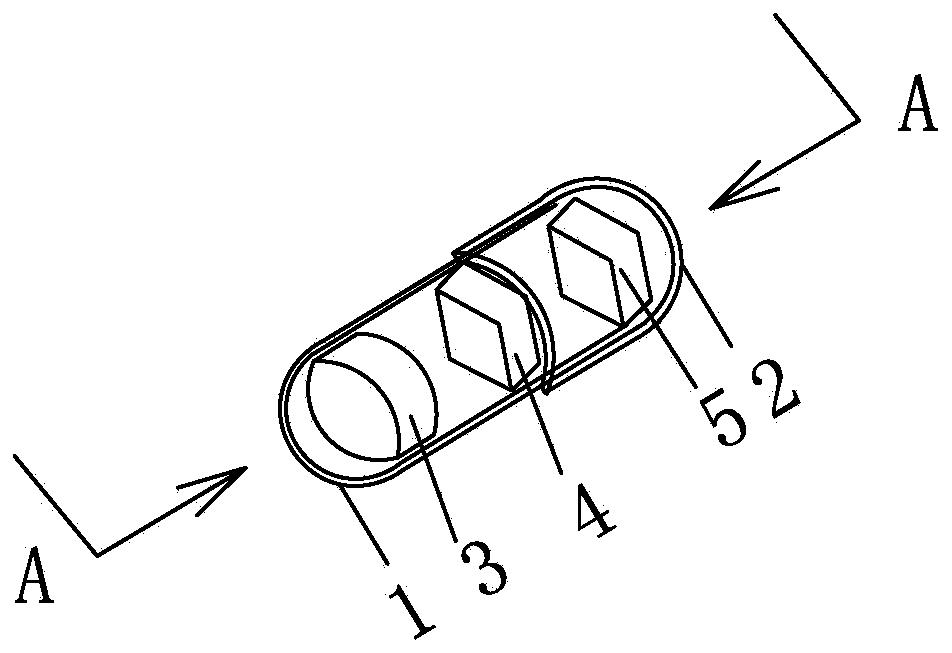
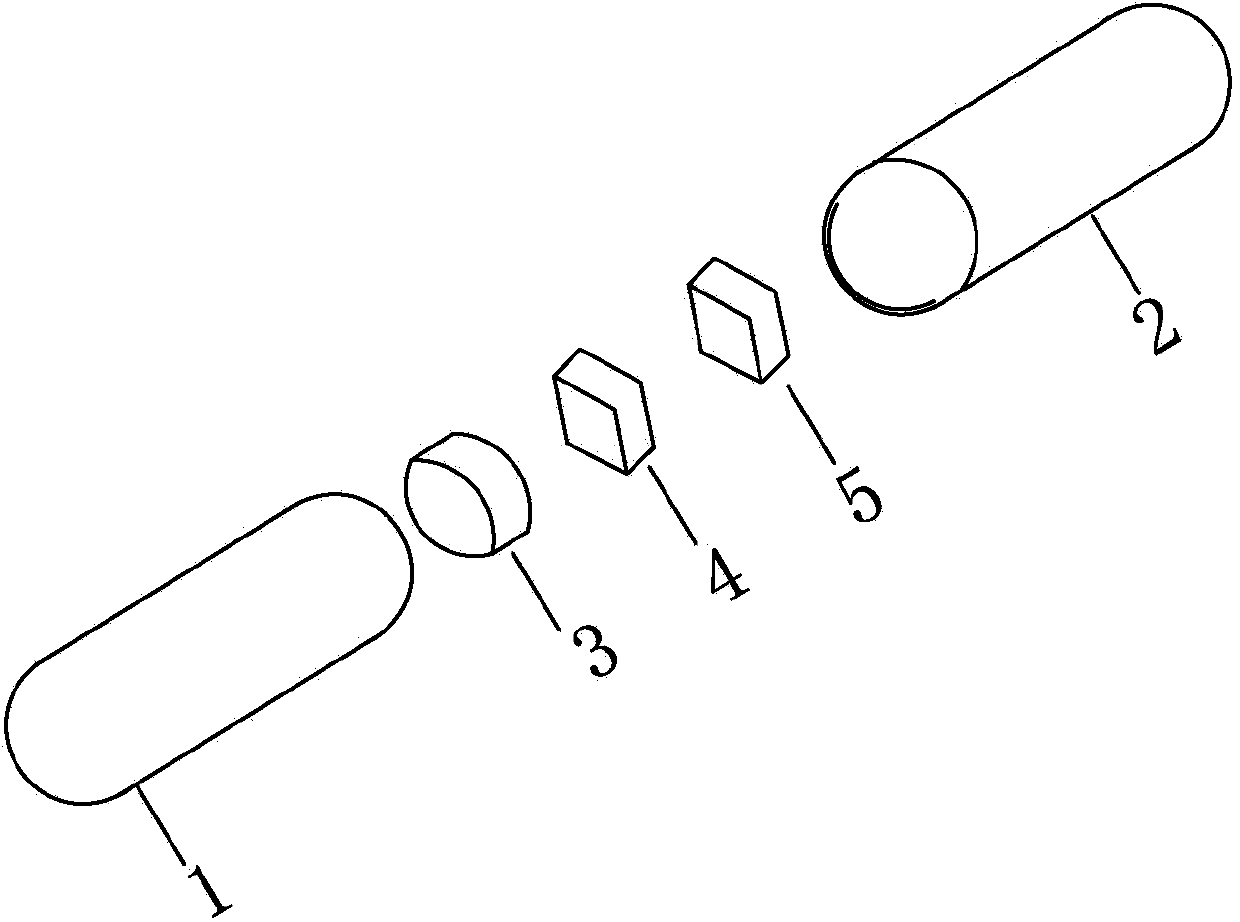
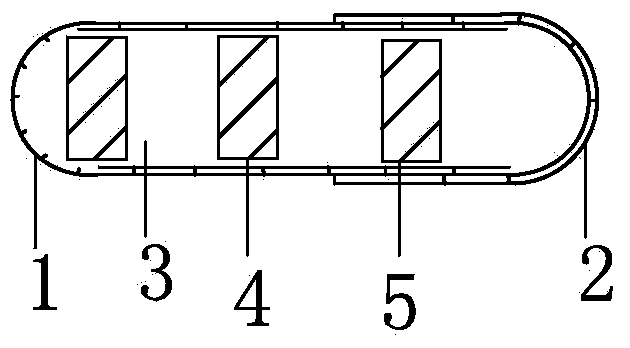
[0027] Embodiment 1: see attached Figure 1-3 As shown, one group of capsules is filled with one 25mg immediate-release topiramate tablet; one 25mg sustained-release topiramate tablet, and one tablet capsule of a 50mg sustained-release topiramate tablet. Capsule specification is No. 3. The capsule shell is composed of a lower capsule body 1 and an upper capsule body 2 set, which are equipped with the above-mentioned 3 tablets of different specifications, which are immediate-release topiramate tablet 3 (containing 25 mg immediate-release topiramate), sustained-release topiramate tablet Tablet 4 (containing extended-release topiramate 25mg) and extended-release topiramate tablet 5 (containing extended-release topiramate 50mg).
[0028] 25mg immediate-release topiramate tablet 3 is a cylindrical red enteric-coated tablet, 25mg sustained-release topiramate tablet 4 is a diamond-shaped red enteric-coated tablet, 50mg sustained-release topiramate tablet 5 is a diamond-shaped green ...
Embodiment 2
[0029] Embodiment 2: basically the same as embodiment 1, a 50mg immediate-release topiramate tablet is filled in one group of capsules; a 25mg sustained-release topiramate tablet, and a tablet capsule of a 50mg sustained-release topiramate tablet. Capsule size is No. 4. The capsule shell is composed of a lower capsule body 1 and an upper capsule body 2 set, which are equipped with the above-mentioned 3 tablets of different specifications, which are immediate-release topiramate tablet 3 (containing 50 mg of immediate-release topiramate) and sustained-release topiramate tablet Tablet 4 (containing extended-release topiramate 50mg) and extended-release topiramate tablet 5 (containing extended-release topiramate 25mg).
[0030] 50mg immediate-release topiramate tablet 3 is cylindrical green enteric-coated tablet, 50mg sustained-release topiramate tablet 4 is diamond-shaped green enteric-coated tablet, 25mg sustained-release topiramate tablet 5 is diamond-shaped red intestinal Dis...
Embodiment 3
[0031] Embodiment 3: basically with embodiment 1, a 25mg immediate-release topiramate tablet is filled in a group of capsules; a 25mg sustained-release topiramate tablet, and a tablet capsule of a 25mg sustained-release topiramate tablet. Capsule specification is No. 3. The capsule shell is composed of a lower capsule body 1 and an upper capsule body 2 set, which are equipped with the above-mentioned 3 tablets of different specifications, which are immediate-release topiramate tablet 3 (containing 25 mg immediate-release topiramate), sustained-release topiramate tablet Tablet 4 (containing extended-release topiramate 25mg) and extended-release topiramate tablet 5 (containing extended-release topiramate 25mg).
[0032] 25mg immediate-release topiramate tablet 3 is cylindrical red enteric-coated tablet, 25mg sustained-release topiramate tablet 4 is diamond-shaped red enteric-coated tablet, 25mg sustained-release topiramate tablet 5 is diamond-shaped red enteric-coated tablet Di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com