New preparation method of febuxostat
A technology of febuxostat and intermediates, which is applied in the field of pharmaceutical synthesis, can solve the problems of unfavorable recovery, etc., and achieve the effects of convenient large-scale production, high yield, and small genotoxic impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
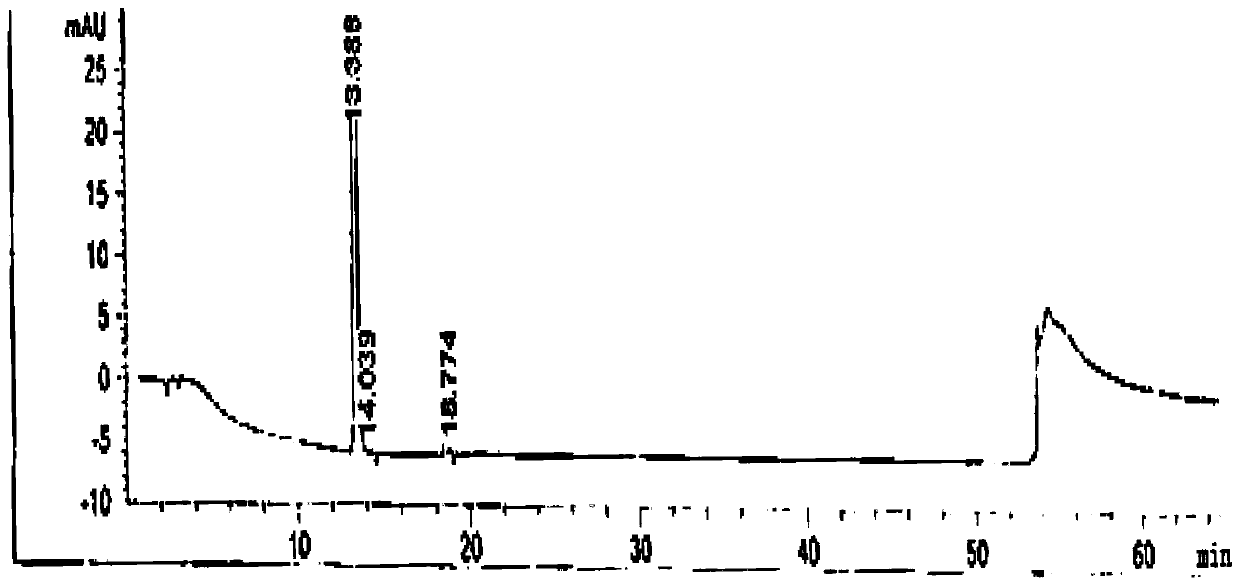
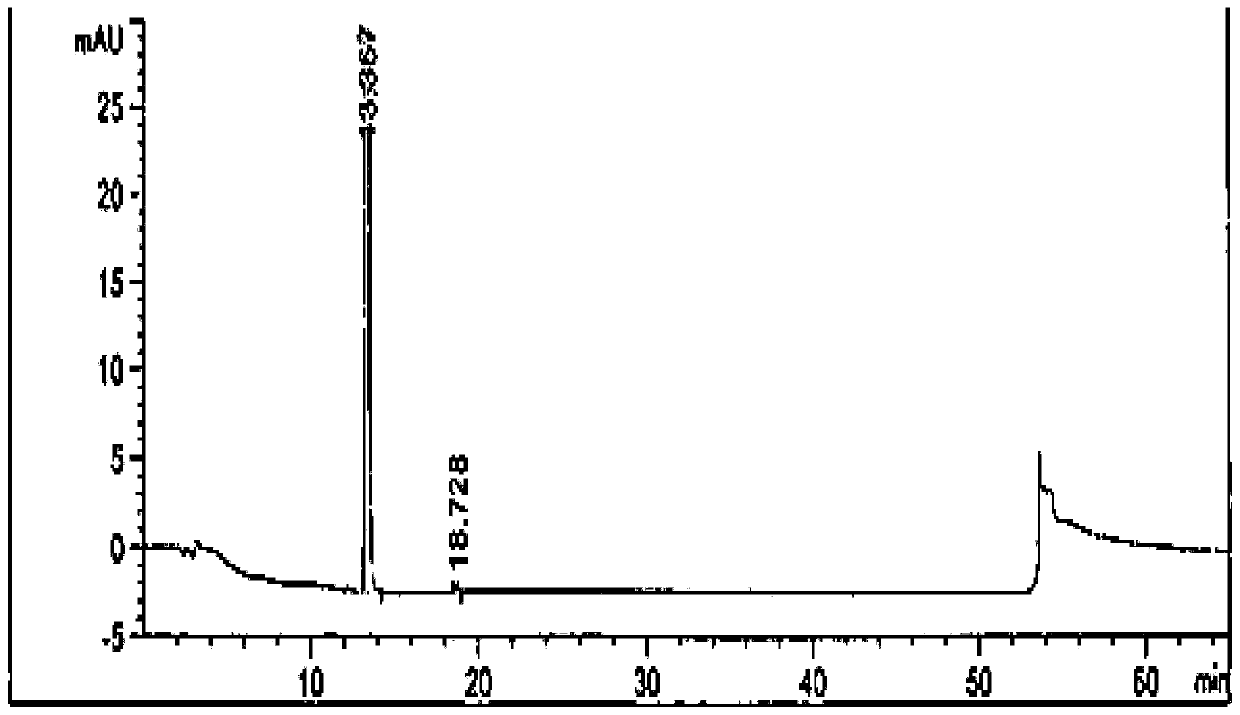
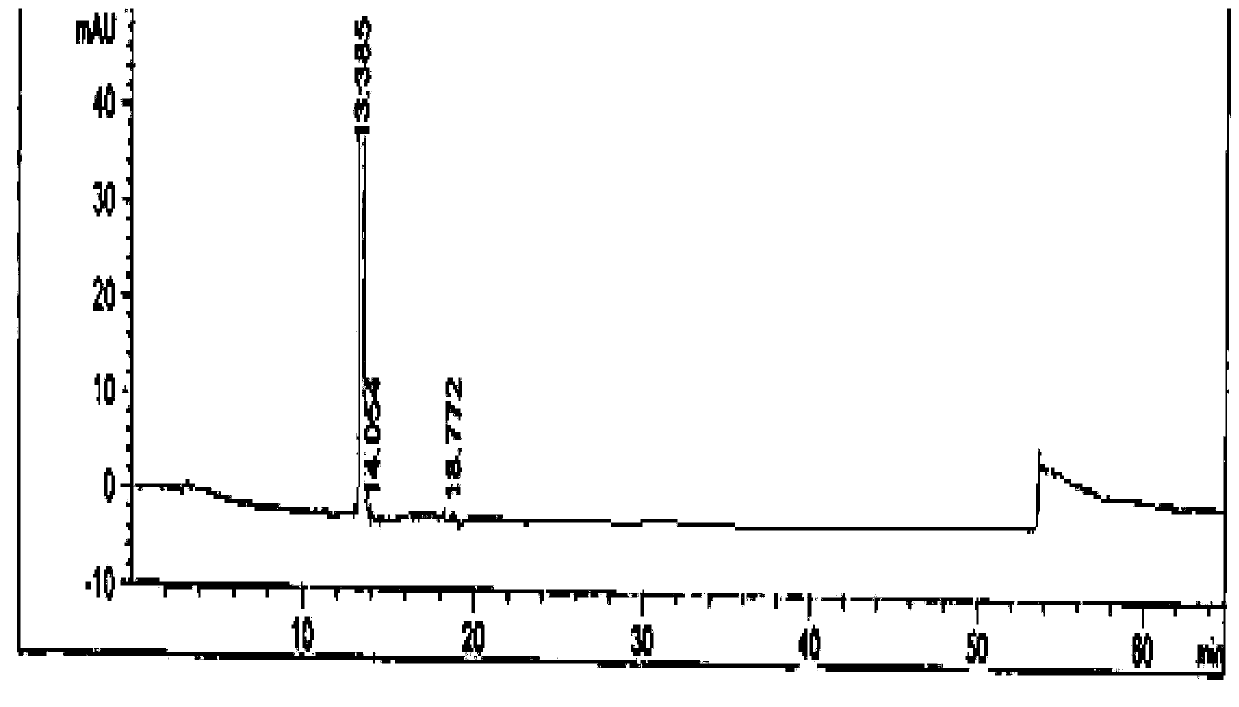
Image
Examples
Embodiment 1
[0066] The preparation of embodiment 1 febuxostat product
[0067] 1. Preparation of crude febuxostat
[0068] Add 400kg DMF, 100kg 2-(3-formyl-4-hydroxyl phenyl)-4-methylthiazole-5-carboxylate ethyl ester, 50kg bromoisobutane, 35kg triethylamine in reaction tank, heat up Reaction at 70°C for 15h. Then cool down and add 25kg of hydroxylamine hydrochloride, and react at 70°C for 3h. Then 30kg of acetyl chloride was added dropwise and reacted at 75°C for 7h. After the reaction is complete, cool down to 20-30°C, add water, add 20kg of sodium hydroxide, and hydrolyze at 40°C for 6 hours. Then hydrochloric acid was added dropwise to adjust the pH to 6.0-7.0. Lower the temperature by 0-5°C, then add water, and crystallize for 2 hours. Centrifuge to obtain a white solid powder, and obtain the febuxostat crude product with a wet weight of 122kg.
[0069] 2. Preparation of febuxostat
[0070] The crude product of febuxostat is dissolved in 90% methanol, decolorized by activated ...
Embodiment 2
[0071] The preparation of embodiment 2 febuxostat products
[0072] 1. Preparation of crude febuxostat
[0073] Add 400kg DMAC, 100kg 2-(3-formyl-4-hydroxyl phenyl)-4-methylthiazole-5-carboxylate ethyl ester, 50kg bromoisobutane, 35kg triethylamine in reaction tank, heat up Reaction at 75°C for 15h. Then cool down and add 25kg of hydroxylamine hydrochloride, and react at 75°C for 3h. Then 30kg of acetyl chloride was added dropwise, and reacted at 80°C for 7h. After the reaction is complete, cool down to 20-30°C, add water, add 20kg of sodium hydroxide, and hydrolyze at 45°C for 6 hours. Then hydrochloric acid was added dropwise to adjust the pH to 6.0-7.0. Lower the temperature by 0-5°C, then add water, and crystallize for 2 hours. Centrifuge to obtain a white solid powder, and obtain the febuxostat crude product with a wet weight of 128kg.
[0074] 2. Preparation of febuxostat
[0075] Dissolve the crude product of febuxostat in 90% methanol, decolorize it with activat...
Embodiment 3
[0076] The preparation of embodiment 3 febuxostat products
[0077] 1. Preparation of crude febuxostat
[0078] Add 400kgDMF, 100kg 2-(3-formyl-4-hydroxyl phenyl)-4-methylthiazole-5-carboxylate ethyl ester, 50kg bromoisobutane, 35kg triethylamine in reaction tank, be warming up to Reaction at 80°C for 16h. Then cool down and add 25kg of hydroxylamine hydrochloride, and react at 80°C for 4h. Then 30kg of acetyl chloride was added dropwise and reacted at 85°C for 8h. After the reaction is complete, cool down to 20-30°C, add water, add 20kg of sodium hydroxide, and hydrolyze at 50°C for 8 hours. Then hydrochloric acid was added dropwise to adjust the pH to 6.0-7.0. Lower the temperature by 0-5°C, then add water, and crystallize for 2 hours. After centrifugation, a white solid powder was obtained, and the powder was extracted to obtain a crude febuxostat product with a wet weight of 132kg.
[0079] 2. Preparation of febuxostat
[0080] Dissolve the crude product of febuxost...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com