Compositions and methods to prevent cell transformation and cancer metastasis
A cancer, transferase technology, applied in the field of cancer diagnosis and treatment, can solve the problems of poorly understood and unsatisfied importance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] The results in this example were obtained from experiments using the materials and methods described in Example 3.
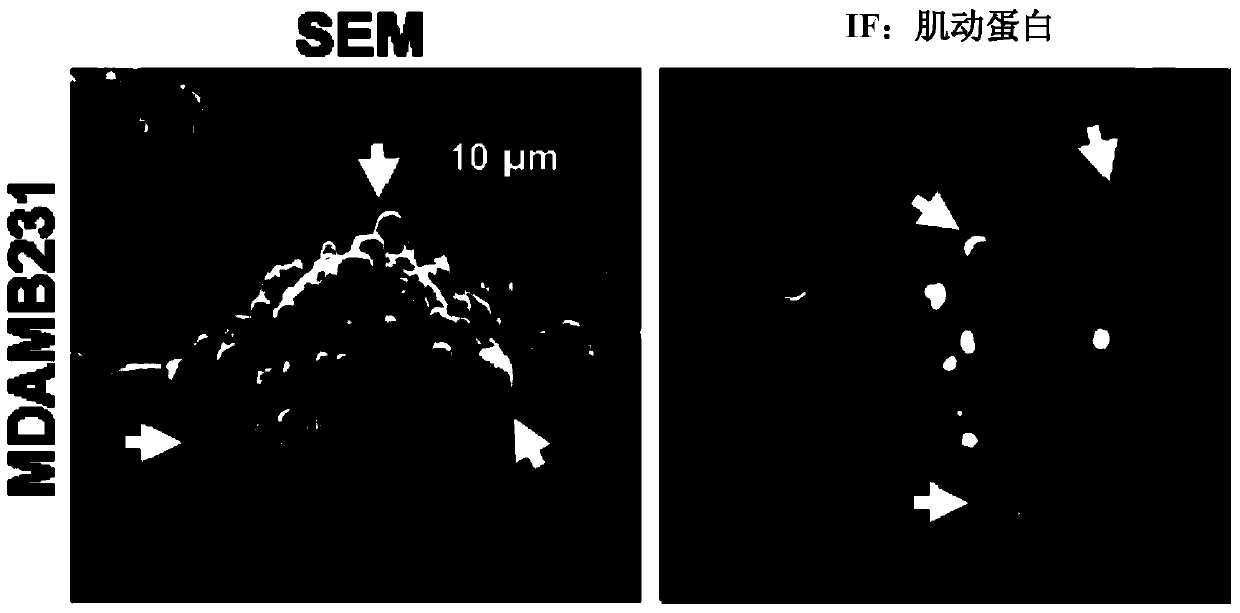
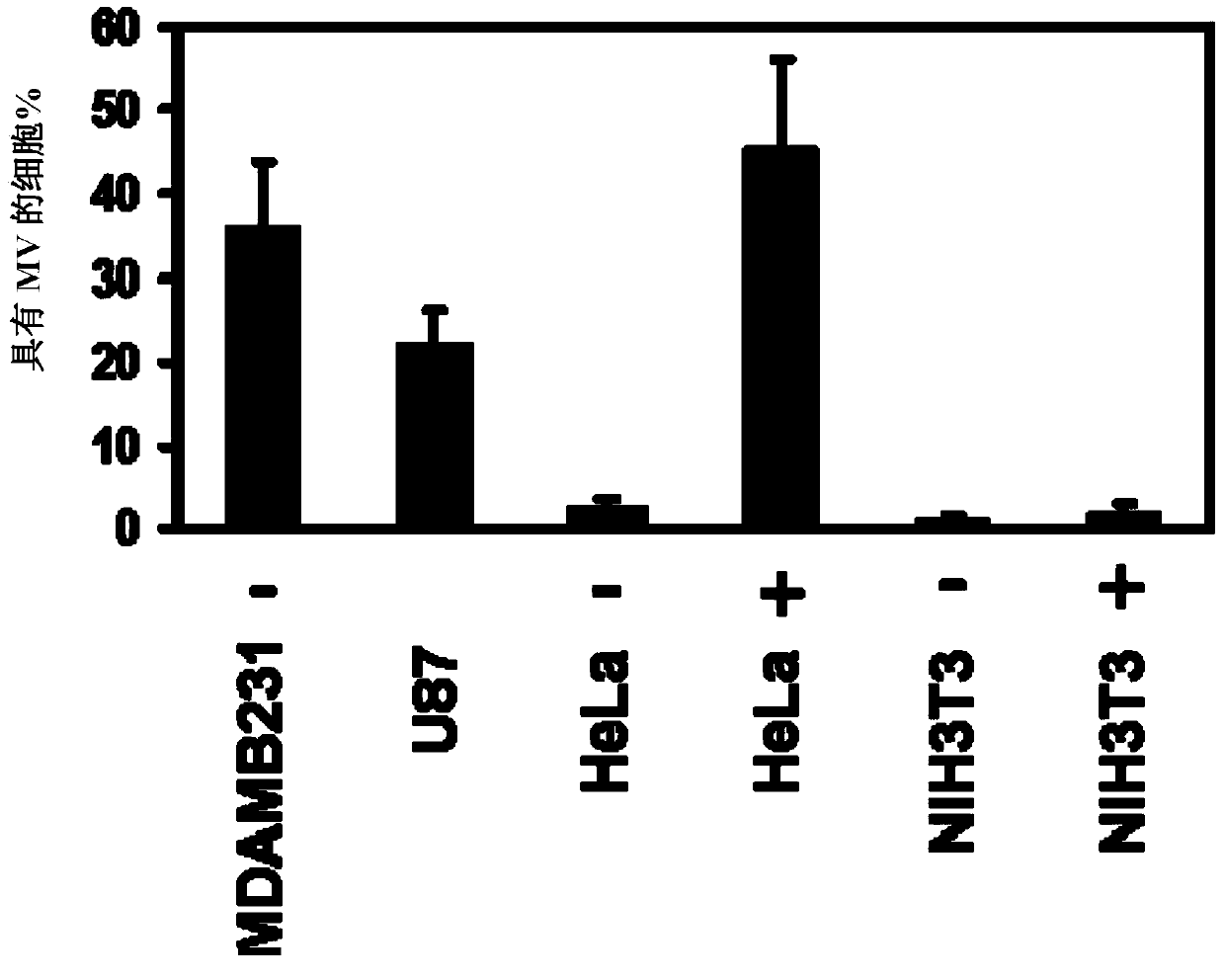
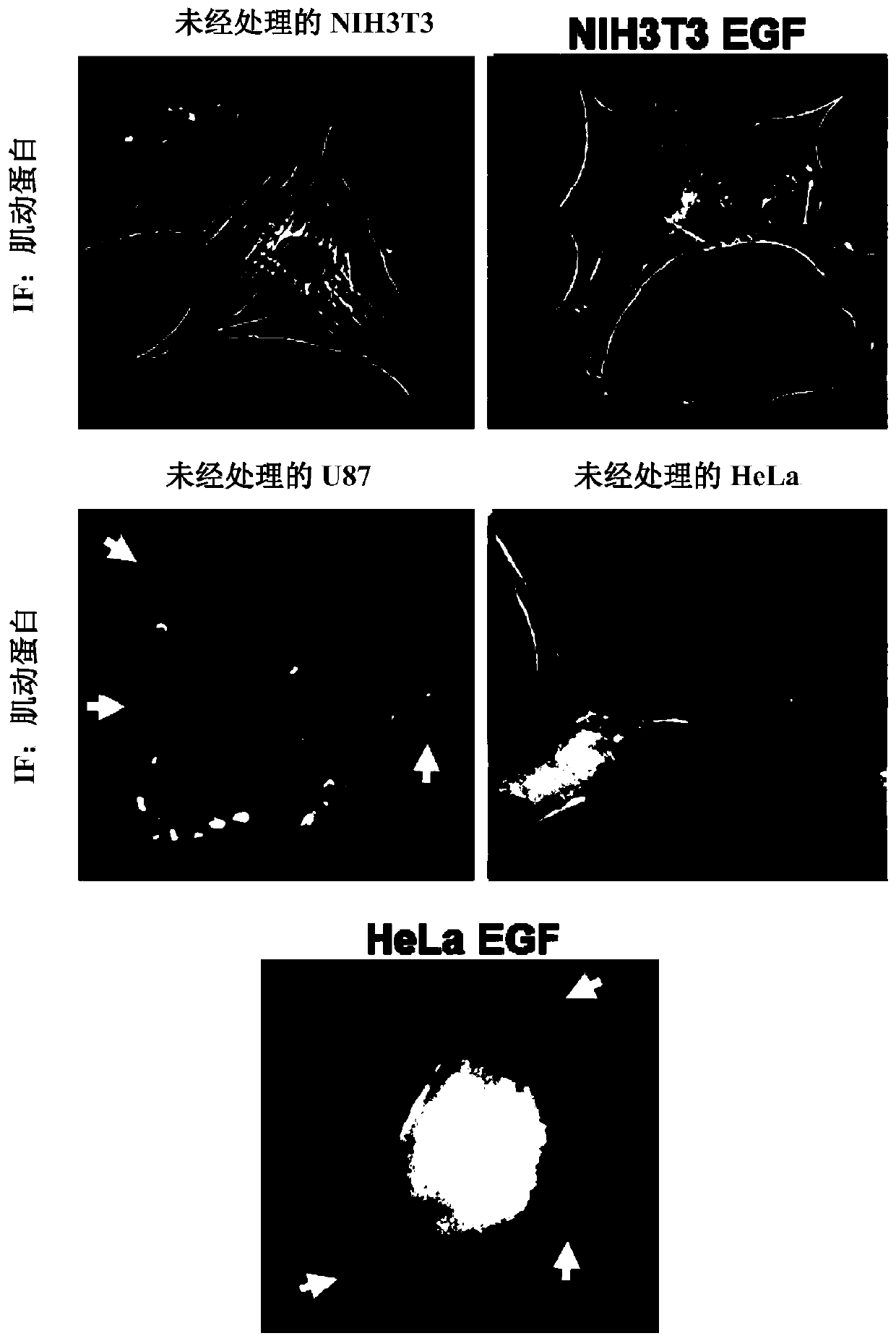
[0117] We used scanning electron microscopy (SEM) ( figure 1 , left panels) or using fluorescence microscopy on cells stained for F-actin ( figure 1 , right) Analysis of serum-starved cultures of the highly invasive human breast cancer cell line MDAMB231 revealed ~35% of the cell surface to have MVs ranging in size from ~0.2-2.0 microns in diameter ( figure 2 ). MVs were also detected on ~25% of serum-depleted U87 human glioma cells, and the formation of these MVs needs to be induced by stimulation with epidermal growth factor (EGF) secreted by HeLa cervical cancer cells ( figure 2 and 3 ). In contrast, MVs were not detected on the surface of normal NIH3T3 fibroblasts cultured under serum-starved or EGF-stimulated conditions, suggesting that some cell types may not produce MVs. Moreover, experiments have shown that MVs are actively shed from these ...
Embodiment 2
[0131] This example describes proteins that co-occur in MVs derived from MDAMB231 cells and U87 cells. Proteomic analysis was performed using MVs shed from MDAMB231 breast cancer cells or U87 brain tumor cells. The following list was compiled from those proteins identified in MVs from MDAMB231 and U87 cells (based on general cellular function): Proteomic analysis of microvesicles shed from MDAMB231 and U87 cells: nucleic acid binding proteins; Nuclear translation elongation factor 1; Eukaryotic translation elongation factor 2; Histone cluster 1; Histone cluster 2; RuvB-like protein 1; RuvB-like protein 2; Extracellular matrix and plasma membrane-associated proteins; Annexin A2; CD9 Antigen; collagen; ecto-5'-nucleotidase; protein 3 containing EGF-like repeats and discoid I-like domains; fibronectin; galectin 3-binding protein; integrin β1; laminin protein; lysyl hydroxylase precursor; major histocompatibility complex; Na+ / K+-ATPase; transglutaminase 2 subtype a; metabolic pro...
Embodiment 3
[0133] This example provides a description of the materials and methods used to obtain the results described in Example 1.
[0134] Material. 4,6-diamidino-2-phenylindole (DAPI), brefeldin A, mitomycin-C, and Exo1 were from Calbiochem, and T101 was from Zedira. Rhodamine-conjugated Phalloidin, EGF, Lipofectamine, Lipofectamine2000, Protein G beads, control and tTG siRNA, and all cell culture reagents were from Invitrogen. FN antibody, MDC, and BPA were from Sigma. tTG and actin antibodies were from Lab Vision / Thermo. Lipid raft marker protein-2 antibody was from Santa Cruz, and HA and Myc antibodies were from Covance. Steriflip PVDF-filters (0.45 μm pore size) were from Millipore. Antibodies against IκBα, GFP, and antibodies recognizing ERK, AKT, FAK, and EGF receptors were from Cell Signaling.
[0135] cell culture. MDAMB231, U87, MCF10A, and HeLa cell lines were grown in RPMI1640 medium containing 10% fetal bovine serum, while NIH3T3 cell lines were grown in DMEM mediu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com