Pharmaceutical application of nafamostat
A technology of nalimus, which is applied in the direction of medical preparations containing active ingredients, drug combinations, pharmaceutical formulas, etc., can solve problems such as hindering the extension of regenerated axons and affecting functional recovery, so as to promote the recovery of motor function and promote survival , the effect of reducing apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Therapeutic Trial of Nafamostat on Spinal Cord Injury
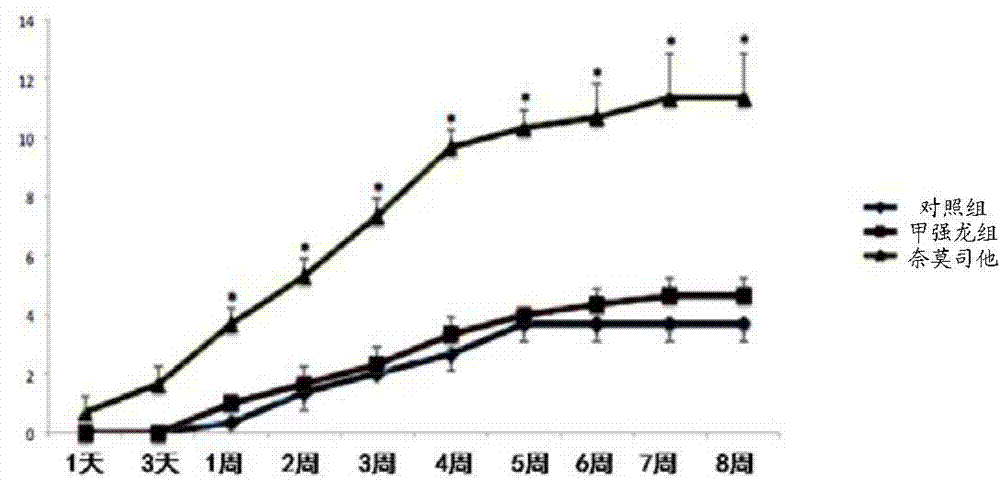
[0030] Using 8-week-old female Wistar rats, the New York University (NYU) standard spinal cord injury impactor (Impactor Model II) was used to impact the spinal cord at the 10th segment of the rat thorax to construct an animal model of spinal cord injury. Eight hours after the injury, Nafamostat was given by intraperitoneal injection at a dose of 10 mg / kg, and methylprednisolone was given by tail vein injection at a dose of 30 mg / kg, once a day until 2 weeks after the injury; Saline served as a control. BBB score, TUNEL, NF-200 and GFAP staining were used to observe the functional recovery, apoptosis after injury, the number of neurons survived and the size of glial scar after treatment.
[0031] Figure 1-4 It shows the comparison of the therapeutic effect of the nafamostat treatment group, the methylprednisolone treatment group and the normal saline control group when the drug was administered 8 hou...
Embodiment 2
[0033] Example 2 Comparison of the therapeutic effects of namostat and methylprednisolone when administered at different times
[0034] In this example, the efficacy of nafamostat for the clinical treatment of spinal cord injury was determined by comparing the curative effects of nafamostat and methylprednisolone when administered at different times.
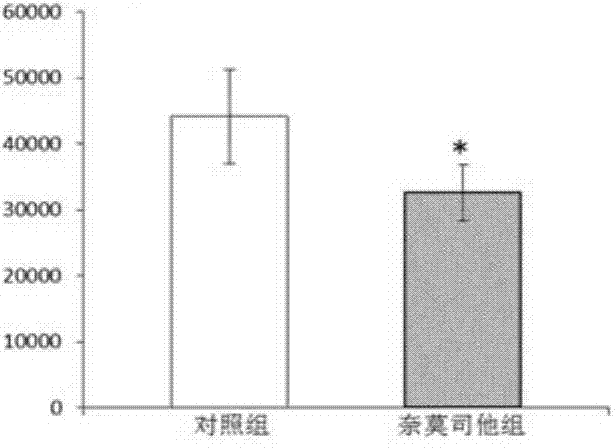
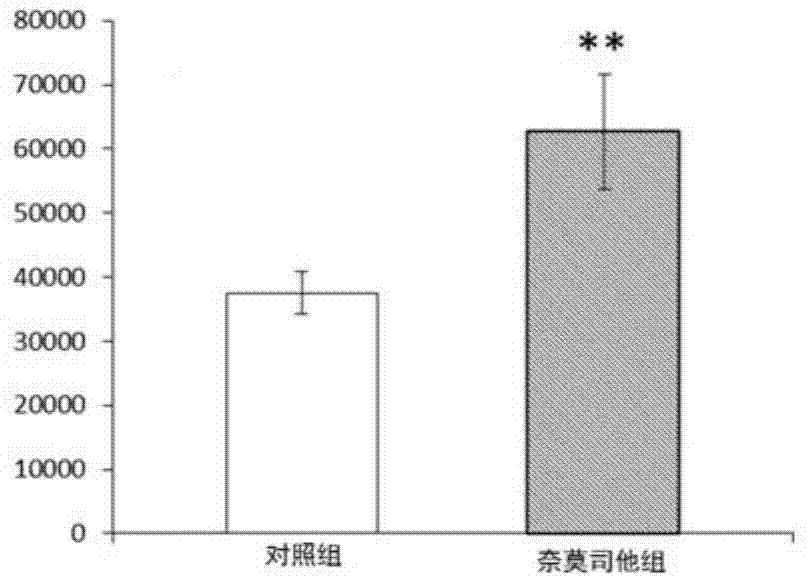
[0035] Eight-week-old female Wistar rats were divided into three groups (30 rats in each group), and the spinal cord was hit at the thoracic 10th segment of the rats with the New York University (NYU) standard spinal cord injury impactor (Impactor Model II). Immediately after the blow was selected ( 0 hour), 3 hours, 8 hours, and 24 hours, the three groups of animals were treated with normal saline, methylprednisolone, and nafamostat for 2 weeks, and the recovery of the rats was observed.
[0036] Figure 5-6 A comparison of the therapeutic effects of the nafamostat treatment group, the methylprednisolone treatment group and ...
Embodiment 3
[0038] Example 3 Nafamostat dosing trial
[0039] In order to determine the optimal dosage of Nafamostat, a gradient concentration of Nafamostat (1mg / kg, 3mg / kg, 10mg / kg, 30mg / kg and 100mg / kg) was designed to treat rats with spinal cord injury. Simple PBS injection group was used as control. Figure 7 The BBB scores of rats treated with different concentrations of nafamostat are shown, from Figure 7 It can be seen that with the increase of the administration concentration, the BBB score increases synchronously, and the BBB score is higher when 10mg / kg, 30mg / kg and 100mg / kg are used. Considering that 10mg / kg, 30mg / kg and 100mg / kg There was no statistically significant difference in the BBB scores of the three groups, and 10 mg / kg was used as the applied dose for animal experiments.
[0040] Based on the above research results, for patients with spinal cord injury, according to the usual 50:1 dose conversion relationship between animals (rats) and humans, 0.2 mg / kg is used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com