Method for preparing beta-Ni(OH)2 flower-like microsphere
A microsphere and flower-like technology, which is applied in the field of preparing β-Ni2 flower-like microspheres, can solve the problems of poor repeatability and achieve the effects of low cost, simple process, and easy large-scale synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1) Dissolve 1.08 g of nickel chloride hexahydrate in 30 ml of ethanol and stir until uniform to obtain a clear solution.
[0017] 2) Transfer the solution prepared in step 1) to a solvothermal device, conduct a solvothermal reaction at 170°C for 6 h, then centrifuge the obtained substance, and then dry it at 80°C to obtain basic nickel chloride powder.
[0018] 3) Disperse 0.1 g basic nickel chloride powder in 20 ml of 3 M potassium hydroxide solution, react for 24 h, wash and dry to obtain β-Ni(OH) 2 Flower-shaped microspheres.
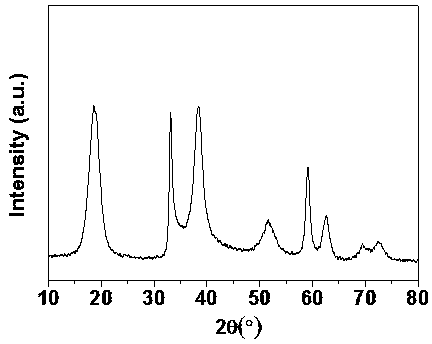
[0019] Prepared β-Ni(OH) 2 The X-ray diffraction pattern is shown in Fig. Its diffraction peaks are similar to those of β-Ni(OH) 2 The standard spectra are consistent, indicating that the obtained product is β-Ni(OH) 2 . Calculated by Scherrer's formula, the average crystal diameter is 4 nm. Prepared β-Ni(OH) 2 The scanning electron microscope photo as figure 2 As shown, it can be seen from the figure that the prepared β-Ni(OH) 2 It ...
Embodiment 2
[0021] 1) Dissolve 1.08 g of nickel chloride hexahydrate in 30 ml of ethanol and stir until uniform to obtain a clear solution.
[0022] 2) Transfer the solution prepared in step 1) to a solvothermal device, perform a solvothermal reaction at 180°C for 9 h, then centrifuge the obtained substance, and then dry it at 80°C to obtain basic nickel chloride powder.
[0023] 3) Disperse 0.1 g basic nickel chloride powder in 20 ml of 6 M sodium hydroxide solution, react for 24 h, wash and dry to obtain β-Ni(OH) 2 Flower-shaped microspheres.
[0024] Prepared β-Ni(OH) 2 The scanning electron microscope photo as image 3 As shown, it can be seen from the figure that the prepared β-Ni(OH) 2 It has a flower-like structure, which is composed of nanosheets.
Embodiment 3
[0026] 1) Dissolve 1.08 g of nickel chloride hexahydrate in 30 ml of ethanol and stir until uniform to obtain a clear solution.
[0027] 2) Transfer the solution prepared in step 1) to a solvothermal device, perform a solvothermal reaction at 130°C for 100 h, then centrifuge the obtained substance, and then dry it at 80°C to obtain basic nickel chloride powder.
[0028] 3) Disperse 0.1 g basic nickel chloride powder in 20 ml of 2 M sodium hydroxide solution, react for 24 h, wash and dry to obtain β-Ni(OH) 2 Flower-shaped microspheres.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com