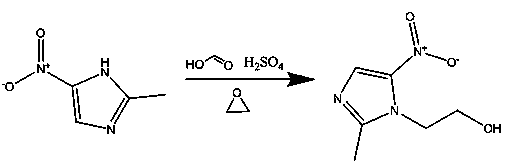Metronidazole production method
A technology for metronidazole and nitroimidazole, applied in the direction of organic chemistry and the like, can solve the problems of large alkali consumption, high cost, large waste water discharge, etc., and achieves reduction of alkali usage, improvement of yield, and reduction of production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
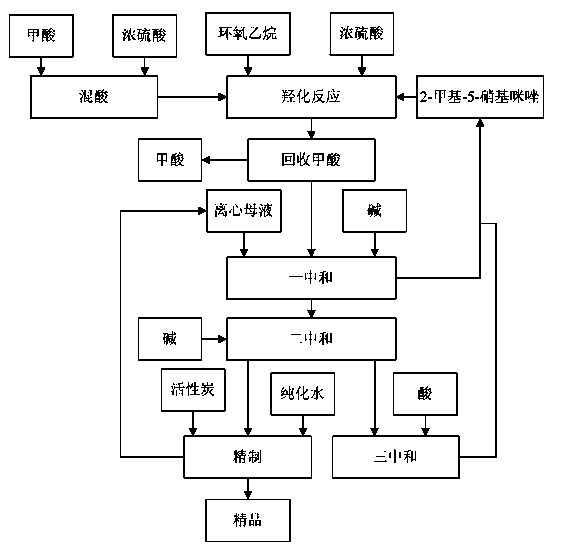
[0032] (1) Preparation of mixed acid: put 270kg of formic acid with a mass concentration of 85% into a hydroxylation reaction kettle, and add 160kg of concentrated sulfuric acid with a mass concentration of 98% while stirring at a temperature of 18°C to prepare a mixed acid.
[0033] (2) Hydroxylation reaction: Put 400kg of 2-methyl-5-nitroimidazole into the hydroxylation reaction kettle equipped with mixed acid, stir and heat up to 80°C, after 2-methyl-5-nitroimidazole is completely dissolved Insulate for 15 minutes, then add 260kg of ethylene oxide and 55kg of 98wt% concentrated sulfuric acid alternately in 240 minutes, and generate hydroxylated liquid after the reaction is completed.
[0034] (3) Recovery of formic acid: transfer the hydroxylated solution obtained in step (2) into a still, control the temperature at 65°C, control the vacuum degree to -0.086MPa, and distill under reduced pressure, the distillate is formic acid.
[0035] (4) Neutralization: transfer the cen...
Embodiment 2
[0040] (1) Preparation of mixed acid: Put 270kg of formic acid with a mass concentration of 90% into a hydroxylation reaction kettle, add 180kg of 98wt% concentrated sulfuric acid while stirring at a temperature of 25°C to prepare a mixed acid.
[0041] (2) Hydroxylation reaction: Put 430kg of 2-methyl-5-nitroimidazole into the hydroxylation reaction kettle equipped with mixed acid, stir and heat up to 88°C, after 2-methyl-5-nitroimidazole is completely dissolved Keep warm for 10 minutes, then add 270kg of ethylene oxide and 60kg of 98wt% concentrated sulfuric acid alternately in 300 minutes, and generate hydroxylated solution after the reaction is completed.
[0042] (3) Recovery of formic acid: transfer the hydroxylated solution obtained in step (2) into a still, control the temperature at 80°C, control the vacuum degree to -0.085MPa, and distill under reduced pressure, the distillate is formic acid.
[0043] (4) Neutralization: Transfer the centrifuged mother liquor obtaine...
Embodiment 3
[0048] (1) Preparation of mixed acid: put 270kg of formic acid with a mass concentration of 88% into the hydroxylation reaction kettle, and add 170kg of 98wt% concentrated sulfuric acid while stirring at a temperature of 32°C to prepare the mixed acid.
[0049] (2) Hydroxylation reaction: Put 420kg of 2-methyl-5-nitroimidazole into the hydroxylation reaction kettle equipped with mixed acid, stir and heat up to 85°C, after 2-methyl-5-nitroimidazole is completely dissolved Insulate for 12 minutes, then add 265kg of ethylene oxide and 56kg of 98wt% concentrated sulfuric acid alternately in 270 minutes, and generate hydroxylated liquid after the reaction is completed.
[0050] (3) Recovery of formic acid: transfer the hydroxylated liquid obtained in step (2) into a distillation kettle, control the temperature at 85°C, control the vacuum degree to -0.090MPa, and distill under reduced pressure, the distillate is formic acid.
[0051] (4) Neutralization: transfer the centrifuged moth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com