Methods of making L-ornithine phenyl acetate
A technology of ornithine phenyl acetate and ornithine salt, which can be used in the fields of medicinal chemistry, biochemistry and medicine, and can solve problems such as excess body fluids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
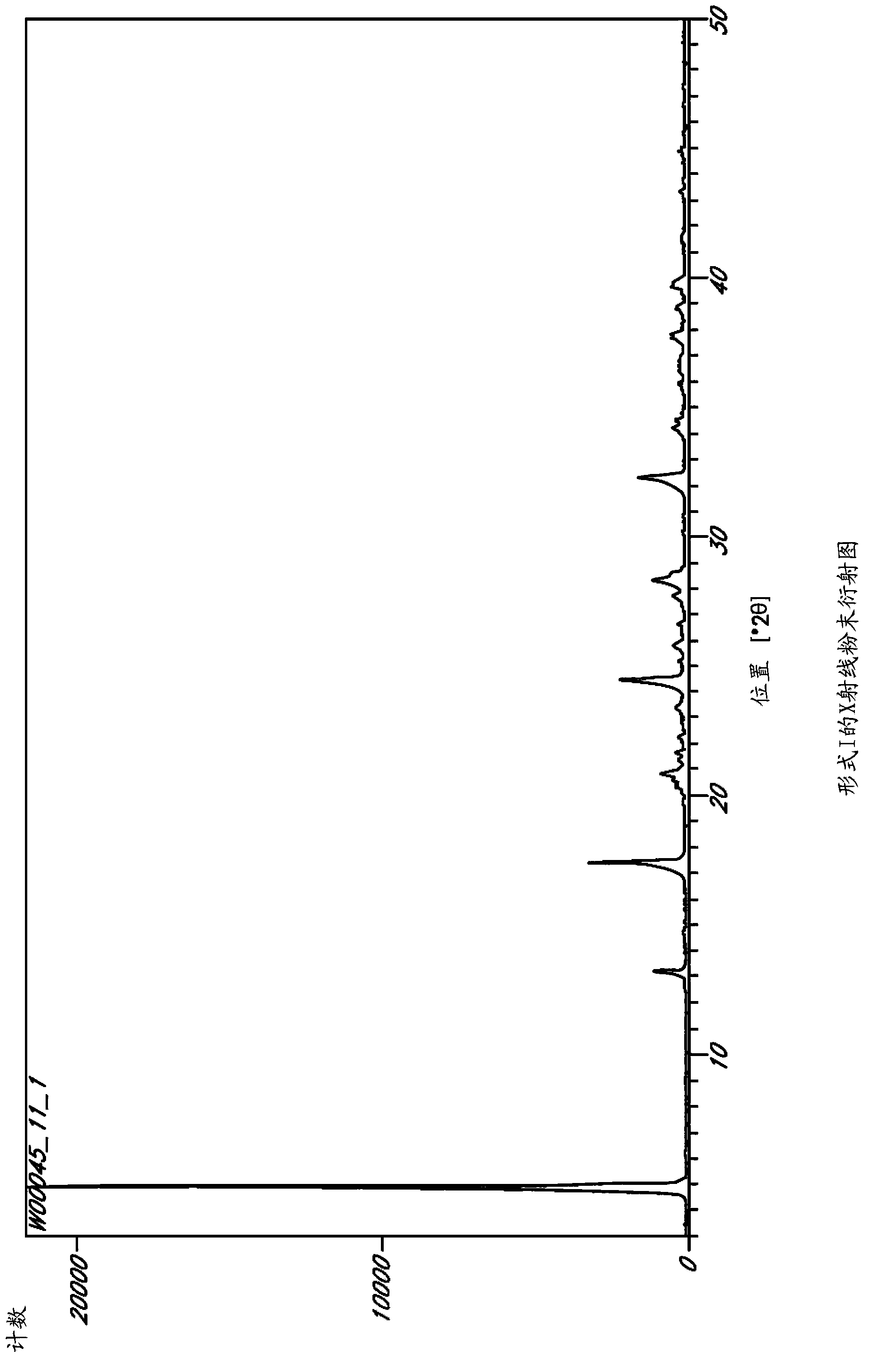
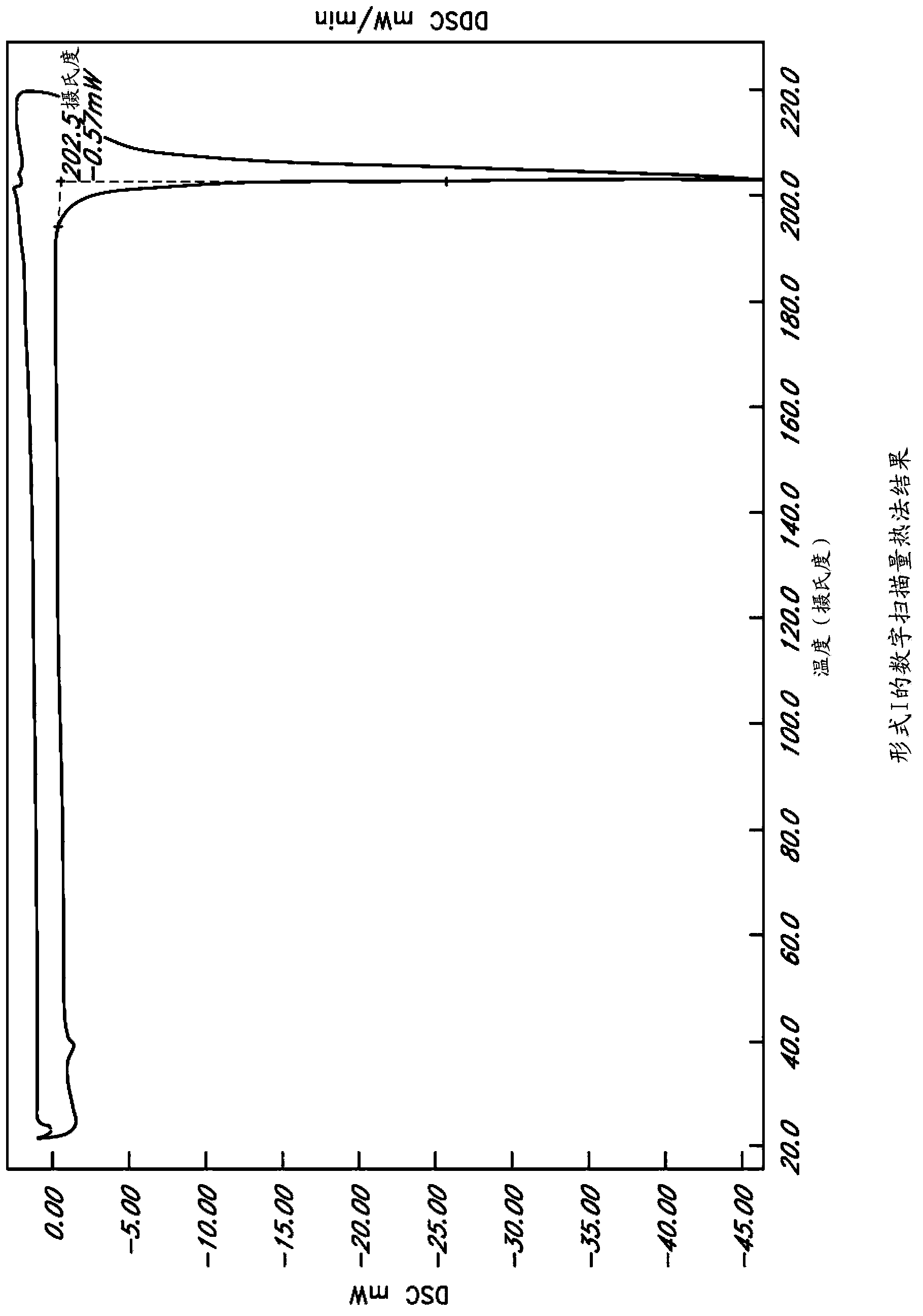
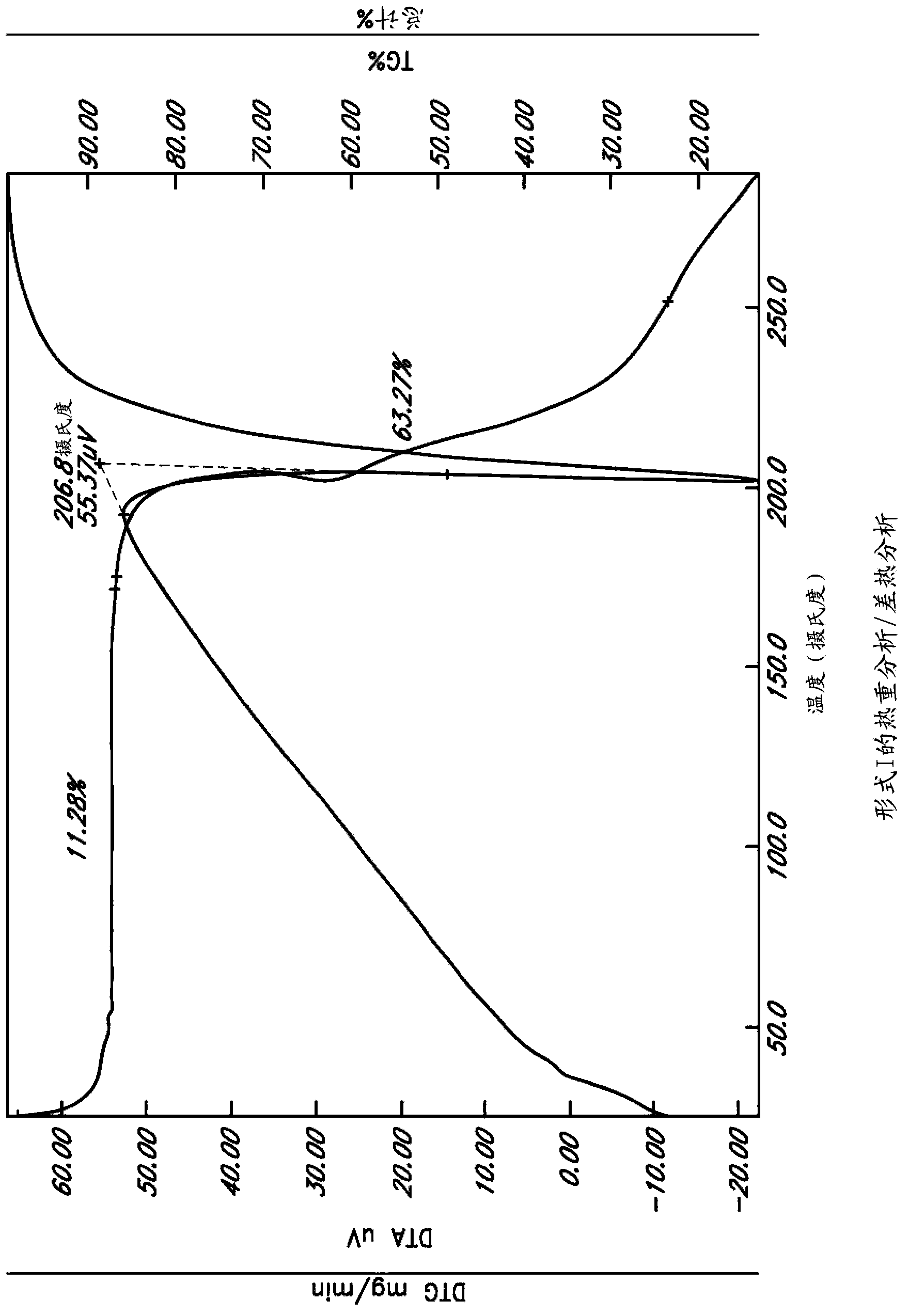
Image
Examples
Embodiment 1
[0181] Example 1: Small scale batch process for the preparation of L-ornithine phenyl acetate
[0182] About 8.4g (0.049mol) of L-ornithine hydrochloride were dissolved in 42mLH 2 O and dissolved approximately 11.4 g of silver benzoate in 57 mL of DMSO. Subsequently, the silver benzoate solution was added to the L-ornithine hydrochloride solution. Combining the two mixtures immediately resulted in the precipitation of an exothermic milky white solid (AgCl). The solids were removed by vacuum filtration and the filtrate was retained (with L-ornithine benzoate in solution). 200 mL of IPA was added to the filtrate and the mixture was cooled to 4°C. A crystalline solid (L-ornithine benzoate) precipitated after about 3 hours and was isolated by vacuum filtration. Yield: 60%.
[0183] Dissolve 7.6 g (0.03 mol) of L-ornithine benzoate in 38 mL of H 2 O and dissolved approximately 4.4 g sodium phenylacetate in 22 mL H 2 O middle. Subsequently, sodium phenylacetate was added t...
Embodiment 2
[0184] Example 2: Large scale batch process for the preparation of L-ornithine phenyl acetate
[0185] Two separate batches of L-ornithine phenylacetate were prepared as follows:
[0186] About 75 kg of L-ornithine monohydrochloride was dissolved in 227 kg of water. To the resulting solution was added 102 kg of silver benzoate dissolved in 266 kg of DMSO at room temperature within 2 hours. A strong exotherm was initially observed and silver chloride precipitated. The receiver containing the solution was then washed with 14 kg of DMSO added to the reaction mass. To remove the silver chloride formed, the reaction mass was filtered with a lens filter made of 10 kg Celite and a 1 mm GAF filter. After filtration, the filter was washed with an additional 75 kg of water. The reaction mass was placed in a separate tank after filtration to prevent contamination with residual silver chloride. The reaction mass was then heated at 35±2° C., and then 80 kg of sodium phenylacetate wa...
Embodiment 3
[0191] Embodiment 3: in-situ preparation sodium phenylacetate solution
[0192] Dissolve phenylacetic acid (PAA) in isopropanol solution. Add about 1 molar equivalent of sodium hydroxide to the solution and stir. The resulting solution was added dropwise to a solution containing about 1 molar equivalent of L-ornithine benzoate. The L-ornithine phenylacetate was precipitated from the solution using generally the same procedure as described in Example 2. Yield: 53.5%. The white powder was further characterized and summarized in Table 6 under the heading "Test A".
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com