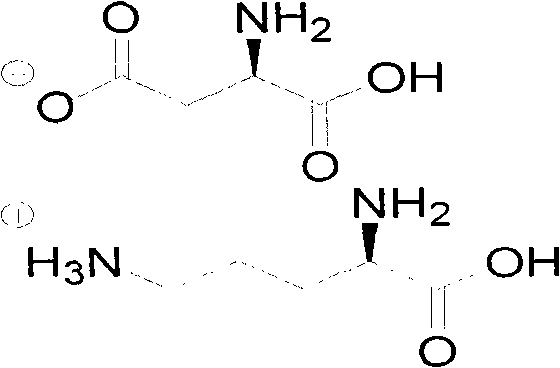
L-ornithine-L-aspartate preparation method
A technology of aspartic acid and ornithine salt, which is applied in the preparation of organic compounds, chemical instruments and methods, and cyanide reaction preparation, etc., can solve the problem of difficult to realize industrialized production, difficult to produce raw medicine preparations, unfavorable industrialized production, etc. problem, to achieve the effect of good product quality, less output of three wastes, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of L-aspartic acid-L-ornithine salt
[0032] The preparation method of L-aspartic acid-L-ornithine salt, comprises the following steps:
[0033] 1. At 20~25℃, add 31.40g (0.1mol) L-ornithine-α-ketoglutarate (containing two molecules of crystal water) into 100ml water, stir to dissolve; add 40.00g (0.3mol) ) L-aspartic acid, add triethylamine, adjust pH7.0~8.0;
[0034] 2. Add 1.57g activated carbon, heat up to 50-55°C, keep warm for 1 hour, and filter with heat;
[0035] 3. Under stirring, drop the filtrate into 300ml of absolute ethanol, crystallize, filter and dry to obtain 23.50g of L-aspartic acid-L-ornithine salt, the yield is 94.6%, and the melting point is 210.4-210.7°C .
[0036] 1 HNMR: 3.75(1H,m), 3.64(1H,t), 2.9(2H,t), 2.66(1H,m), 2.53(1H,m), 1.78(2H,m), 1.64(1H,m) , 1.58 (1H, m).
[0037] Content: 100.17% ROI: 0.02% [α] D 20 =+28.20°
Embodiment 2
[0038] Example 2 Preparation of L-aspartic acid-L-ornithine salt
[0039] 1. At 20~25℃, add 45.60g (0.1mol) L-ornithine citrate to 140ml of water, stir to dissolve; add 40.00g (0.3mol) L-aspartic acid, pass ammonia gas , adjust pH7.0~8.0;
[0040] 2. Add 2.30g activated carbon, heat up to 50-55°C, keep warm for 0.5 hours, and filter with heat;
[0041] 3. The filtrate was dropped into 420ml of absolute ethanol under stirring, crystallized, filtered and dried to obtain 25.35g of L-aspartic acid-L-ornithine salt, the yield was 95.6%, and the melting point was 208.8-210.9°C .
[0042] Content: 100.74% ROI: 0.03% [α] D 20 =+28.13°
Embodiment 3
[0043] Example 3 Preparation of L-aspartic acid-L-ornithine salt
[0044] 1. At 20~25℃, add 40.00g (0.1mol) L-ornithine malate to 120ml water, stir to dissolve; add 40.00g (0.3mol) L-aspartic acid, add sodium ethoxide, Adjust pH7.0~8.0;
[0045] 2. Add 2.00g activated carbon, heat up to 50-55°C, keep warm for 0.5 hours, and filter with heat;
[0046] 3. The filtrate was dropped into 360ml of absolute ethanol under stirring, and the product was separated, filtered, and dried to obtain 24.53g of L-aspartic acid-L-ornithine salt, the yield was 92.5%, and the melting point was 210.5-211.10 ° C .
[0047] Content: 100.69%; ROI: 0.02%; [α] D 20 =+28.07°
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com