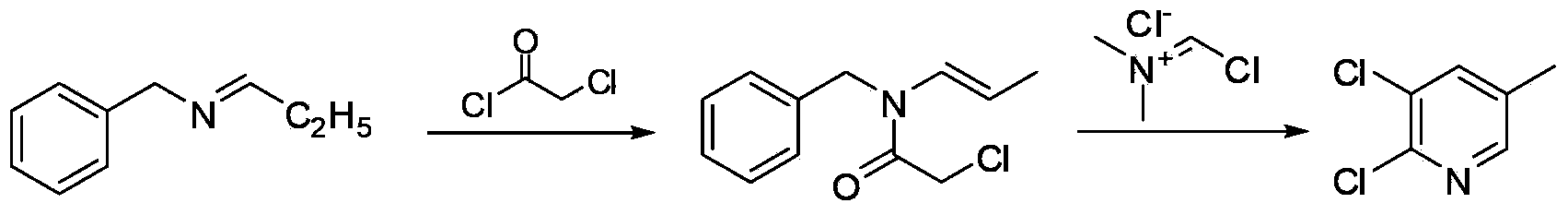
Synthesis method of 2,3-dichloro-5-methylpyridine
A technology of picoline and benzyl, which is applied in the field of synthesis of 2,3-dichloro-5-picoline, can solve the problems of high cost, low yield, and high toxicity of raw materials, and achieve low cost and high yield High and low toxicity of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the preparation of N-benzyl-N-propenyl-alpha-chloroacetamide
[0019] Add 73.5g (0.50mol) of N-propylidenebenzylamine, 100mL of ethyl acetate and 55.6g (0.55mol) of triethylamine into a 500mL four-neck flask, stir and cool down to 0°C, and dropwise add 59.3g of chloroacetyl chloride ( 0.525mol), the dropwise addition was completed within 1-2 hours, then the temperature was raised to 30°C and the reaction was stirred for 1 hour, and the reaction was complete as detected by GC-MS. The reaction liquid was washed successively with 100 mL of water, 50 mL of 10% hydrochloric acid, 50 mL of 10% sodium hydroxide solution, and 50 mL of saturated brine, and then distilled off the solvent under reduced pressure to obtain 109.6 g of viscous liquid, N-benzyl-N-propenyl-α - Chloroacetamide content 96.1%, yield 94.3%.
Embodiment 2
[0020] Embodiment 2: Preparation of N-benzyl-N-propenyl-α-chloroacetamide
[0021] Add 73.5g (0.50mol) of N-propylidene benzylamine, 100mL tetrahydrofuran and 47.4g (0.60mol) of pyridine into a 500mL four-neck flask, stir and cool down to 0°C, add 62.2g (0.55mol) of chloroacetyl chloride dropwise, After 1-2 hours, the dropwise addition was completed, then the temperature was raised to 10°C and the reaction was stirred for 3 hours, and the reaction was detected by GC-MS to be complete. The reaction liquid was washed successively with 100 mL of water, 50 mL of 10% hydrochloric acid, 50 mL of 10% sodium hydroxide solution, and 50 mL of saturated brine, and then distilled off the solvent under reduced pressure to obtain 111.3 g of a viscous liquid, N-benzyl-N-propenyl-α - Chloroacetamide content 95.9%, yield 95.5%.
Embodiment 3
[0022] Embodiment 3: Preparation of 2,3-dichloro-5-picoline
[0023] Add 9.8g (0.058mol) of dimethylchloromethylene ammonium chloride and 100mL of 1,2-dichloroethane into a 250mL three-necked flask, stir and cool down to 10°C, and the N-benzyl- Dissolve 11.1g (0.048mol) of N-propenyl-α-chloroacetamide in 50mL of 1,2-dichloroethane, slowly add it dropwise into the reaction flask, control the temperature within 50°C, raise the temperature to 50°C after the addition, The reaction was incubated for 8 hours, and the reaction was detected by GC-MS to be complete. The reaction solution was washed successively with 100 mL of water, 50 mL of 10% sodium hydroxide and 50 mL of water, and the solvent was distilled off under reduced pressure to obtain an oily liquid, which was then recrystallized by adding 30 mL of n-hexane to obtain 6.6 g of colorless crystals, 2,3-dichloro-5 -The picoline content is 99%, and the yield is 84.1%. M.p.41-43℃, GC-MS (m / z): 161 (100), 126, 98, 90, 73, 63; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com