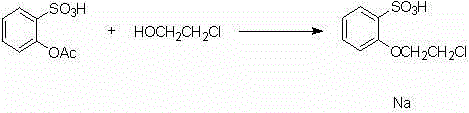Green Synthesis Process of Tribesulfuron-methyl for Broadleaf Weed Removal in Wheat Fields
A broadleaf agent ether, synthetic process technology, applied in the direction of organic chemistry, etc., can solve problems such as large environmental pressure, poisoning, employee injury, etc., and achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Take a 250mL clean round bottom flask, add 100mL N,N-dimethylformamide, add 9.2g 2-acetoxybenzenesulfonic acid, 4.83g 2-chloroethanol, 5.95g thionyl chloride, 5.05g Triethylamine was stirred and reacted for a period of time. Add 3.65g n-butylamine, 2.05g acetonitrile, 2g sodium hydroxide. After stirring and reacting for a period of time, after concentration, the product was obtained by column chromatography
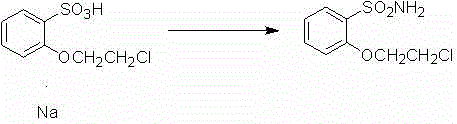
[0019] (2) Take a 250mL round bottom flask, add 10.23g 2-(1-chloroethoxy)-benzenesulfonic acid, and add 100mL chlorobenzene solution. Add 9.9g of phosgene, and control the temperature of the oil bath at 85-105°C. Add 0.73g of N,N-dimethylformamide, and react for 5 hours and then the temperature drops to 60-70°C. 11.3 g of 30% ammonia solution was added within 1 hour and stirring was continued for 2 h. After cooling, the precipitate was filtered off.
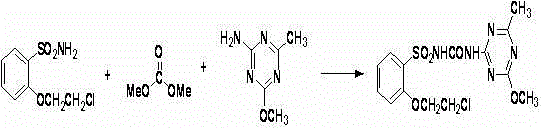
[0020] (3) Take a 250mL round bottom flask, add 14g 2-amine-4-methoxy-6-methyl-1,3,5-triazine, 5.4g sodium...
Embodiment 2
[0021] Example 2 (1) Take a 5000mL clean round bottom flask, add 200mL N,N-dimethylformamide, add 18.4g 2-acetoxybenzenesulfonic acid, 9.66g 2-chloroethanol, 11.9g thionyl chloride , 10.1g triethylamine was stirred and reacted for a period of time. Add 7.3g n-butylamine, 4.1g acetonitrile, 4g sodium hydroxide. After stirring and reacting for a period of time, after concentration, the product was obtained by column chromatography
[0022] (2) Take a 500mL round bottom flask, add 20.46g 2-(1-chloroethoxy)-benzenesulfonic acid, and add 200mL chlorobenzene solution. Add 19.8g of phosgene, and control the temperature of the oil bath at 85-105°C. Add 1.46g of N,N-dimethylformamide, and react for 5 hours and the temperature drops to 60-70°C. 22.6 g of 30% ammonia solution were added within 1 hour and stirring was continued for 2 h. After cooling, the precipitate was filtered off.
[0023] (3) Take a 500mL round bottom flask, add 28g 2-amine-4-methoxy-6-methyl-1,3,5-triazine, 10....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com