Montelukast sodium pulse release preparation
A Montelukast sodium and pulse technology, applied in respiratory diseases, pharmaceutical formulations, block delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
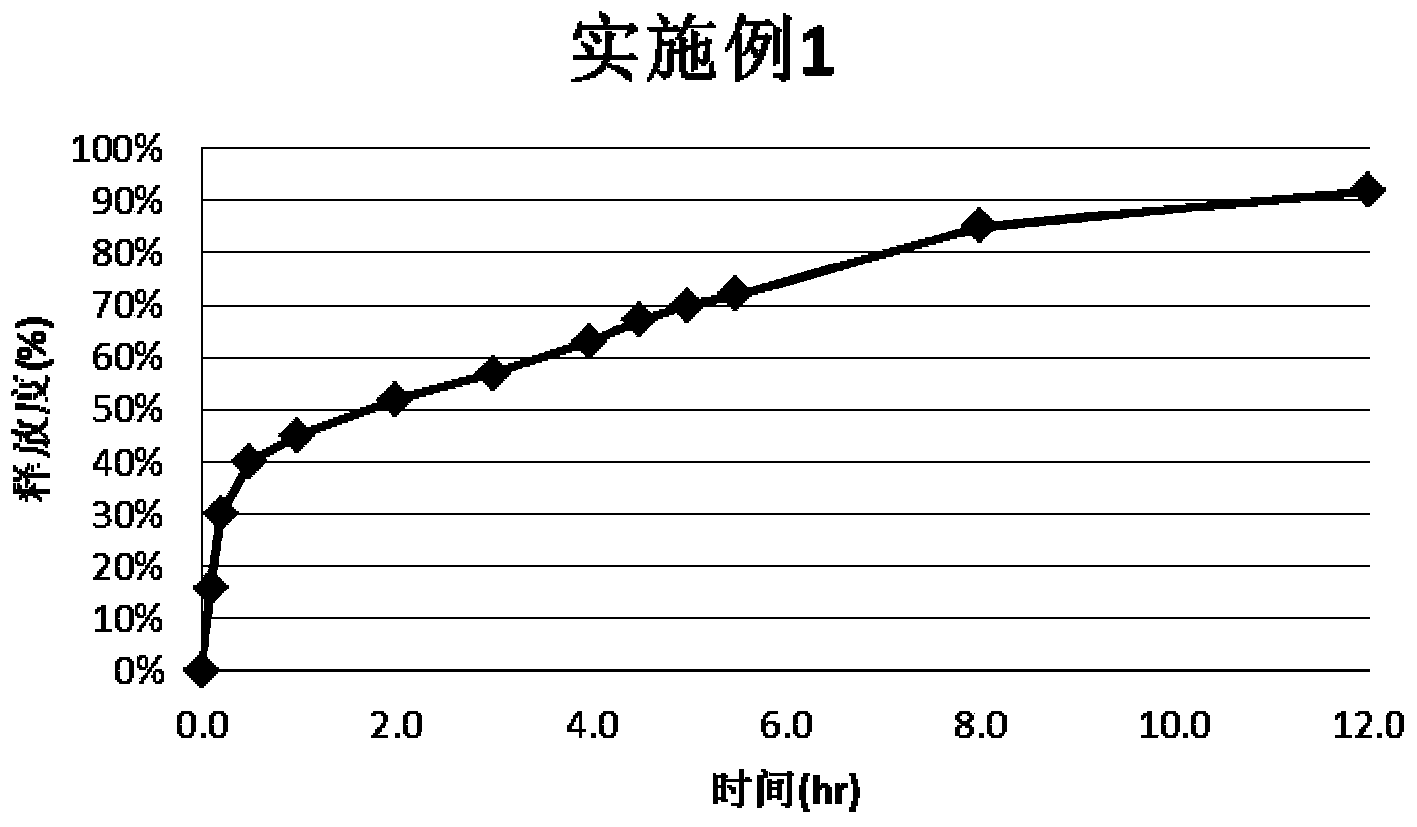
Embodiment 1
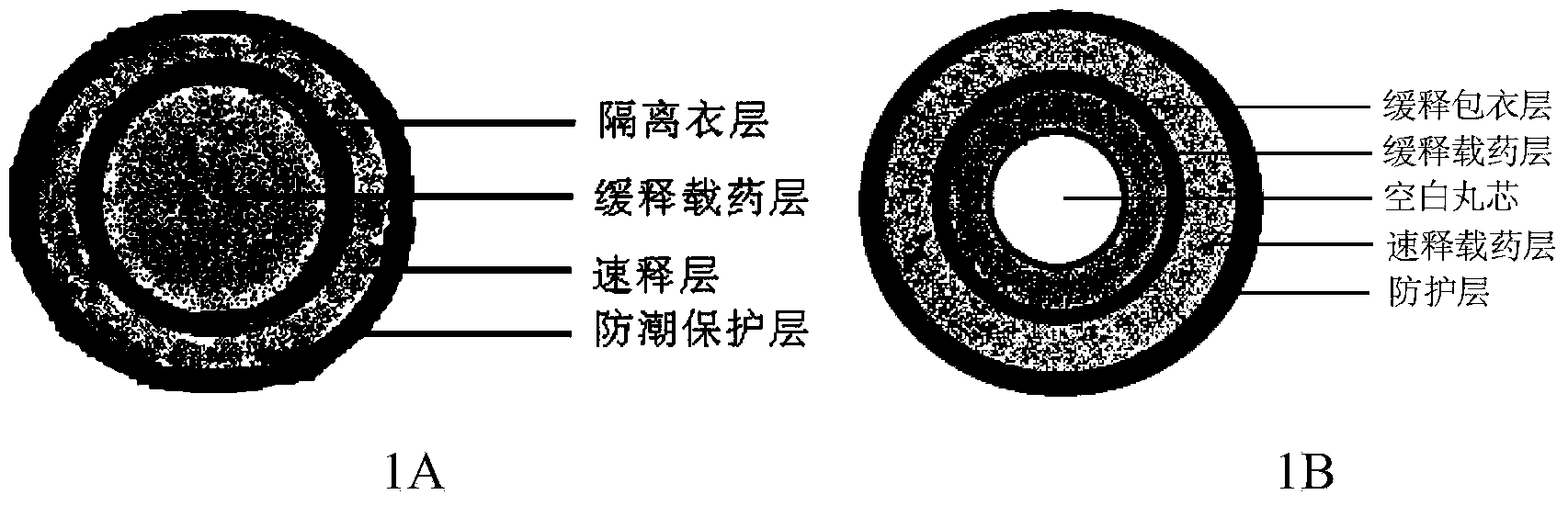
[0024] Take 33g of montelukast sodium and 220g of lactose, place in a wet method, mix for 180 seconds, then add 550g of microcrystalline cellulose, and mix for 180 seconds; take 587g of ethyl cellulose aqueous dispersion (ECD30), add sebacic acid di 44g of butyl ester, stirred for 120 minutes and then added to the wet granulator under stirring to produce soft material; then the soft material was extruded through a 0.6mm screen, and then rounded. Transfer the wet pellets to a fluidized bed for drying, set the inlet air temperature at 60°C, dry until LOD<3.0%, discharge, and sieve to remove sticky and fine particles.
[0025] Take 930g of the aforementioned finished pellets and transfer them to a fluidized bed; take 70g of Opadry Clear, add water to prepare a 15% coating solution, spray it into the fluidized bed, and keep the temperature of the material at 38°C to 45°C; after coating, continue to dry until LOD<3.0%.
[0026] Take 20g of Montelukast Sodium and 20g of HPC EF, add...
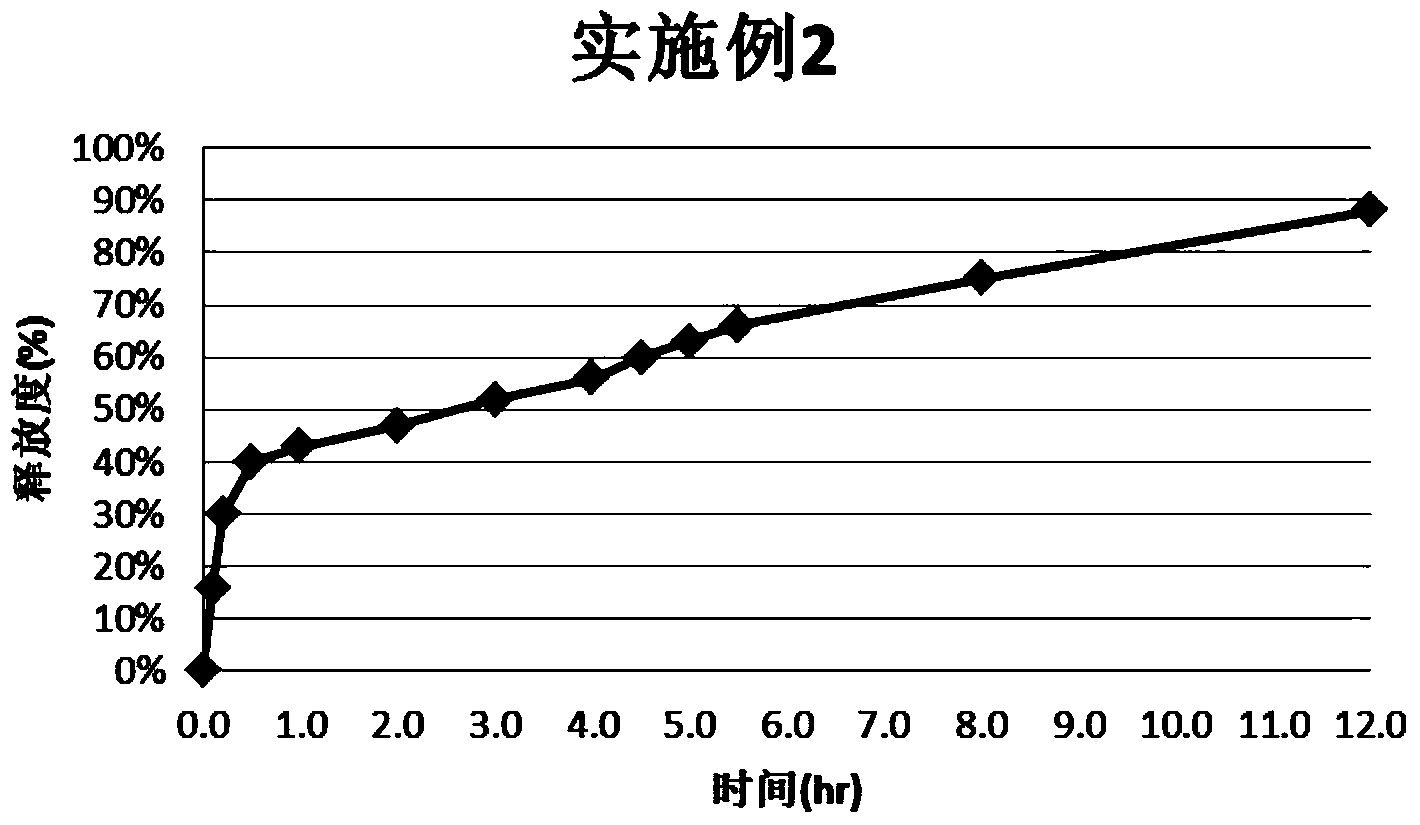
Embodiment 2
[0028] Take 800g blank pellet core and put it in the fluidized bed; take 30g of montelukast sodium raw material and HPMC E530g, add 540g of water, stir well and spray into the fluidized bed, keep the temperature of the material at 38°C-45°C, and finish spraying After that, continue to dry until LOD SR30D170g, NE30D267g, GMS6g, Tween803g, add appropriate amount of water, make a coating solution with a solid content of 15%, continue to spray into the fluidized bed, control the temperature of the material at 25 ° C ~ 28 ° C, after spraying, add 1% hard pellets Calcium fatty acid, continue to dry, make the material temperature reach 38 ℃ ~ 42 ℃, keep for 30 minutes.
[0029] Take 20g of Montelukast Sodium and 20g of HPC EF, add 360g of water, stir to fully dissolve; put the above-mentioned pellets in a fluidized bed, spray the above-mentioned Montelukast Sodium and HPC EF suspension, and keep the material temperature at 35°C ~45°C; after spraying, take 60g of Opadry White, add w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com