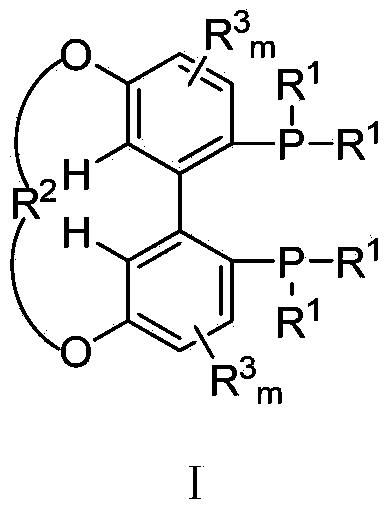
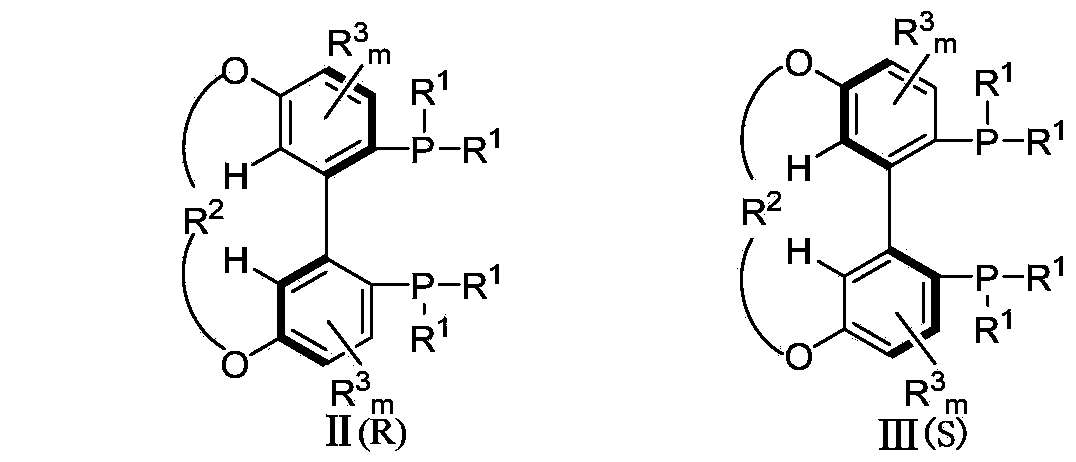
5,5'-connected 1,1'-biphenyl axially chiral 2,2'-diphosphine ligand and preparation method thereof
A bisphosphine ligand and axial chirality technology, which is applied to the 1,1`-biphenyl-like axial chiral 2,2'-bisphosphine ligand connected at the 5,5` position and the field of preparation thereof, which can solve the influence of Asymmetric catalytic reaction catalytic activity and selectivity to achieve high catalytic performance and enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
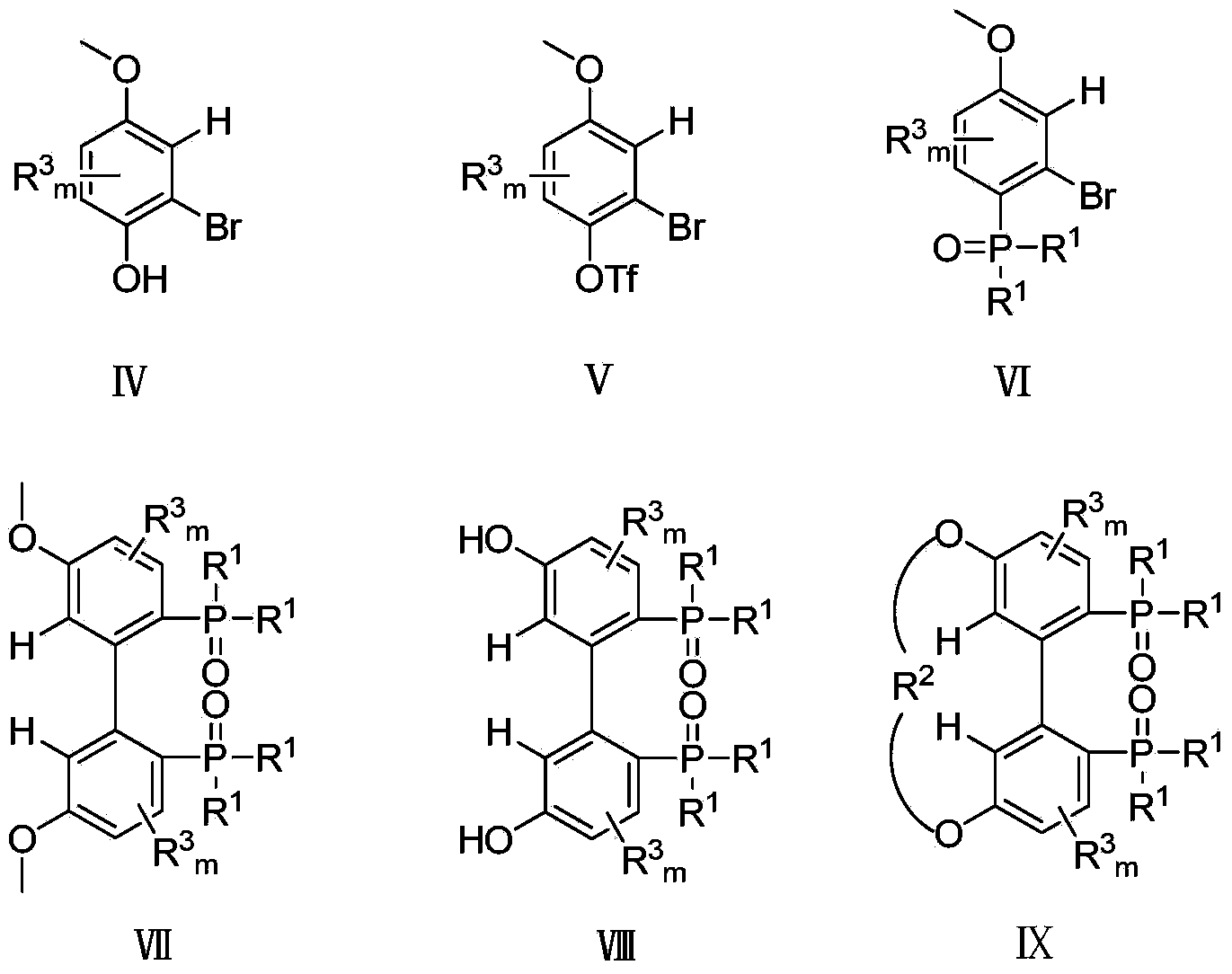
[0038] Example 1: Preparation of compound IV from 4-methoxyphenol
[0039] 4-Methoxyphenol (1.24 g, 10 mmol) dissolved in CH 2 Cl 2 (60ml) solution, slowly drop Br at room temperature 2 (0.51ml, 10.1mmol), continued to stir for 30min, and filtered to obtain compound IV (1.84g, 91%).
[0040] 1 H NMR (400MHz, CDCl 3)δ7.25(d,J=10Hz,1H,ArH),7.18(d,J=3Hz,1H,ArH),6.88(dd,J=10,3Hz,1H,ArH),3.82(s,3H, OMe).
Embodiment 2
[0041] Embodiment 2: Preparation of compound V from compound IV
[0042] Compound IV (2.01g, 10mmol) and pyridine (2.88ml, 35mmol) were dissolved in CH 2 Cl 2 (20ml), add Tf dropwise under ice bath 2 O (4.87ml, 28.7mmol), after the dropwise addition was completed, the temperature was naturally raised and stirred overnight, and 1N HCl (2ml) was added under ice bath and stirred for 1h, the organic layer was separated, and Na 2 SO 4 Dry and spin dry to obtain product V (3.11 g, 93%).
[0043] 1 H NMR (400MHz, CDCl 3 )δ7.25(d,J=10Hz,1H,ArH),7.18(d,J=3Hz,1H,ArH),6.88(dd,J=10,3Hz,1H,ArH),3.82(s,3H, OMe).
Embodiment 3
[0044] Embodiment 3: Preparation of compound VI from compound V
[0045] Compound V (1.68g, 5mmol), diphenylphosphine hydrogen (1.21g, 6mmol), diisopropylethylamine (1.31ml, 7.5mmol), Pd 2 (dba) 3 .CHCl 3 (0.23g, 0.25mmol), dppp (0.11g, 0.25mmol) were dissolved in toluene (10ml), refluxed for 11h, extracted with EtOAc, Na 2 SO 4 Dried, spin-dried, and passed through the column with ethyl acetate and petroleum ether to obtain product VI (1.10 g, 57%).
[0046] 1 H NMR (400MHz, CDCl 3 ) δ7.69(m,4H,ArH),7.55(m,2H,ArH),7.47(m,4H,ArH),7.27(dd,J=12,8Hz,1H,ArH),7.21(t,J =2Hz,1H,ArH),6.82(dq,J=8,3,2Hz,1H,ArH),3.82(s,3H,OMe).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com