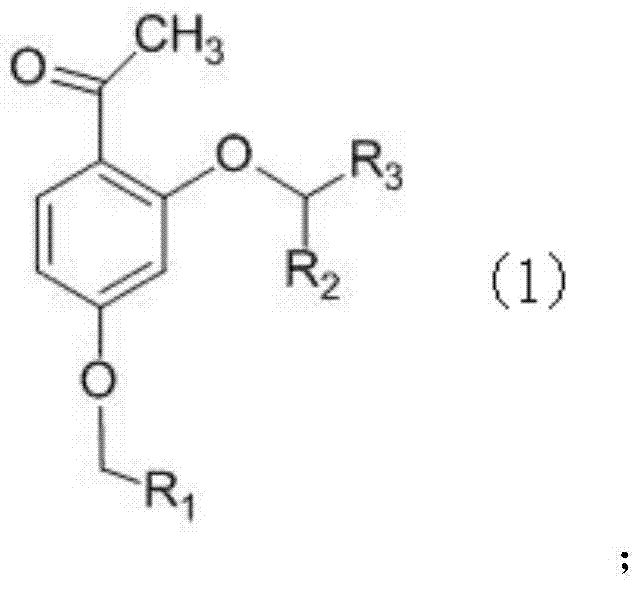
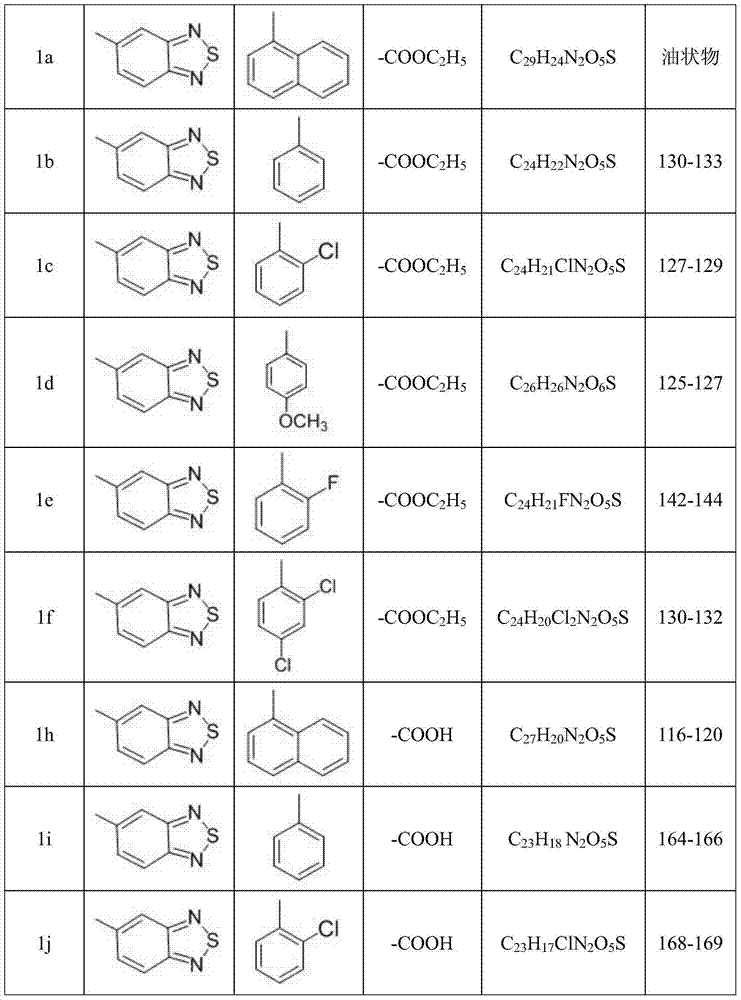
Phenoxy phenylacetic acid endothelin antagonist, and preparation method and application thereof
A technology of phenoxyphenylacetic acids and endothelin antagonists, which is applied in the field of medicine and chemical industry, can solve the problems of low oral bioavailability of peptide antagonists and limit the application of cardiovascular diseases, and achieve strong endothelin receptor antagonistic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The synthesis of embodiment 1 α-bromonaphthalene ethyl acetate (3a)
[0057] Put 3.7g (0.02mol) of naphthaleneacetic acid into a 250mL eggplant-shaped bottle, dissolve it in 50mL of absolute ethanol, add 2mL of concentrated sulfuric acid, heat to reflux for 2 hours, and then cool. Then 2 / 3 of the excess ethanol was evaporated, 100 mL of water was added, the pH was adjusted to 7 with NaHCO3, extracted with ethyl acetate, the organic layer was washed twice with saturated brine, and dried over anhydrous magnesium sulfate. Filter off anhydrous magnesium sulfate, concentrate to obtain a light yellow oil, dissolve in 100mL CCl4, add 3.9g NBS and a small amount of benzoyl peroxide (BPO), heat and reflux for 4 hours, cool, filter, concentrate to obtain a yellow oil, 4.6 g, 80% yield in two steps.
Embodiment 2
[0058] Example 2 Preparation of ethyl 4-methoxyphenyl α-chloroacetate (3d)
[0059] (1) Preparation of 4-methoxyphenyl α-glycolic acid
[0060] Dissolve 6ml (0.05mol) anisaldehyde in 16ml chloroform, add 1g TABA, stir and heat to reflux. At 56°C, 50% NaOH solution was added dropwise, and a khaki solid was formed. The reaction process temperature is controlled at 60-65°C. After the reaction is complete, add distilled water to dissolve and acidify with dilute HCl. Extracted with ethyl acetate, anhydrous MgSO 4 dry. Filter, evaporate to dryness, and recrystallize from toluene to obtain 6.4 g of 4-methoxyphenyl α-glycolic acid, with a yield of 70.3%.
[0061] (2) Preparation of ethyl 4-methoxyphenyl α-hydroxyacetate
[0062] Add 4.1g (0.023mol) of 4-methoxyphenyl α-glycolic acid, 50ml of ethanol, add 2ml of concentrated sulfuric acid, heat to reflux, evaporate excess ethanol, add 100mL of water, use NAHCO3 to adjust the pH to neutral, and then acetic acid extracted with eth...
Embodiment 3
[0065] Example 3 Synthesis of 2-hydroxyl-4-(2,1,3-benzothiadiazole 5-methoxy)acetophenone (2a)
[0066] (1) Synthesis of 5-methyl-2,1,3-benzothiadiazole
[0067] Add 46.5g (0.5mol) of freshly distilled aniline and 250ml of toluene to a 500ml three-necked flask, and slowly add 39.5ml (0.55mol) of SOCl dropwise under ice-cooling and stirring. 2 and 50ml of toluene, after the addition was completed, it was refluxed for 1 hour, at which point the reaction solution became a clear transparent solution. Cool to room temperature, add 30.5g (0.25mol) of 3,4-diaminotoluene, reflux for 6 hours under stirring, cool to room temperature, wash with dilute hydrochloric acid, saturated sodium bicarbonate solution and water successively, and dry over anhydrous magnesium sulfate. Filtrate, evaporate the solvent under reduced pressure to obtain a light yellow oily liquid, put it in the refrigerator for several hours, and precipitate white needle-like crystals, filter the yellow residual liquid w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com