Nitric oxide donor endothelin receptor antagonist as well as preparation method and application thereof
An endothelin receptor and nitric oxide technology, which is applied in the field of medicine and chemical industry, can solve the problems of restricting the application of cardiovascular diseases, low oral bioavailability of peptide antagonists, etc., and achieves significant therapeutic effect and strong endothelin receptor antagonism. active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
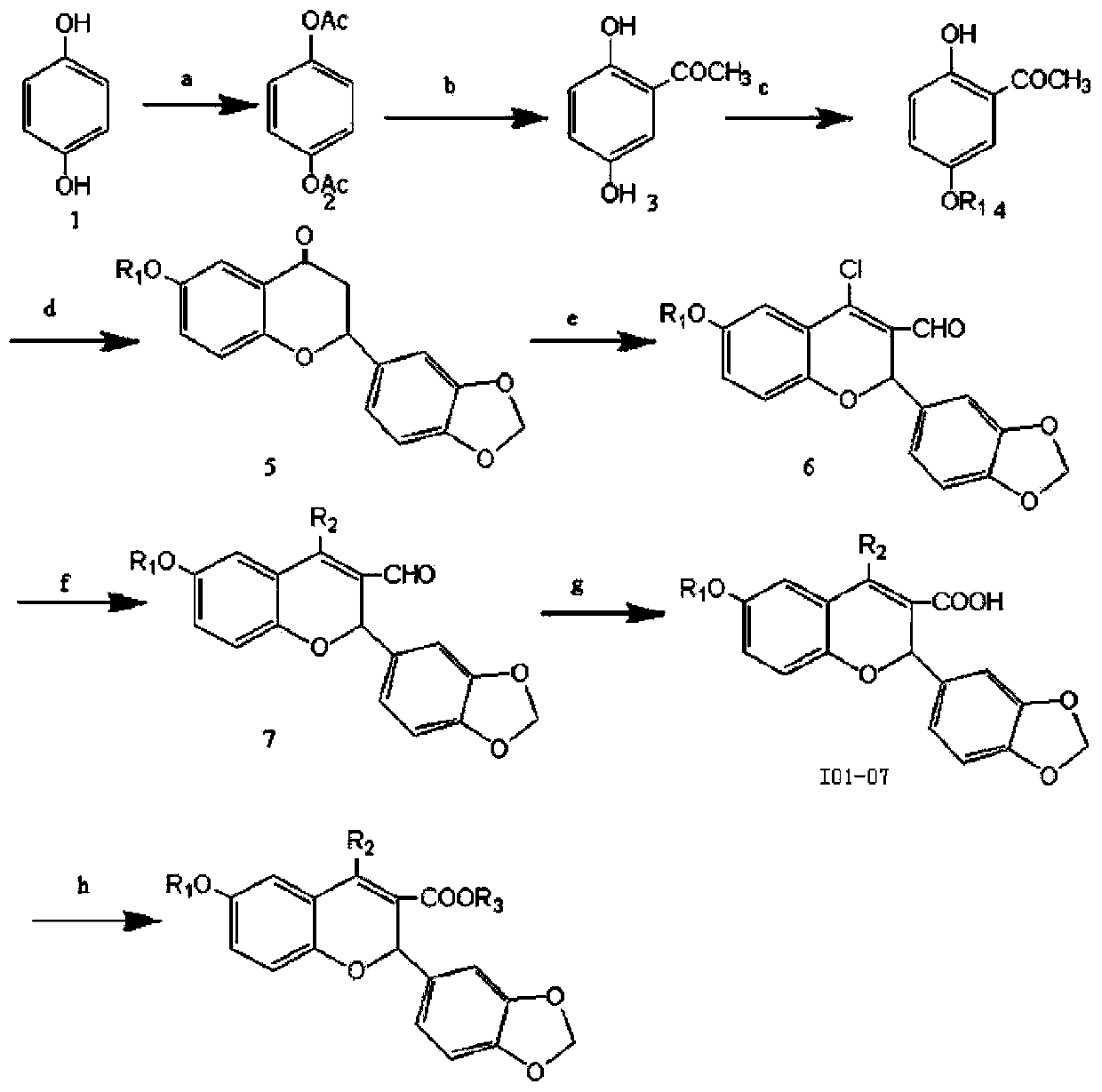
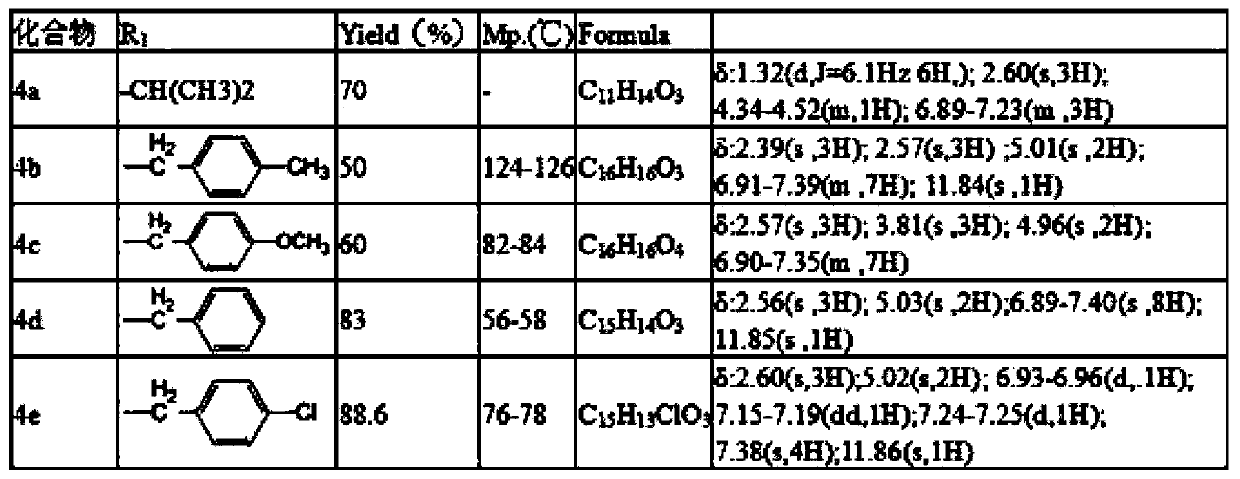
[0020] Preparation of 5-isopropoxy-2-hydroxyacetophenone: Weigh 15.2 grams (0.1mol) of 2,5-dihydroxyacetophenone, 36.92 grams (0.3mol) of 2-bromopropane, 3.7 grams of potassium iodide, 16.6 Add gram (0.12mol) of potassium carbonate to a 150mL conical flask, then add 60mL of acetone, reflux for 24 hours under stirring, filter, spin the filtrate, and pass the residue through the column (petroleum ether: ethyl acetate = 10:1 mixed solvent was the eluent), and 13.6 grams of yellow oil was obtained, with a yield of 70%; the preparation of 5-benzyloxy-2-hydroxyacetophenone: weigh 1.9 grams (12.5 mmol) of 2,5 - Dihydroxyacetophenone, 3.45 grams (25 mmol) of potassium carbonate, 1.6 grams (12.5 mmol) of benzyl chloride, and 3.7 grams of potassium iodide were added to a 150 mL ground-mouth Erlenmeyer flask, and then 60 mL of acetone was added, slowly dripping Add an acetone solution containing 1.6 g (12.5 mmol) of benzyl chloride, after the addition is complete, reflux for 24 hours und...
Embodiment 1
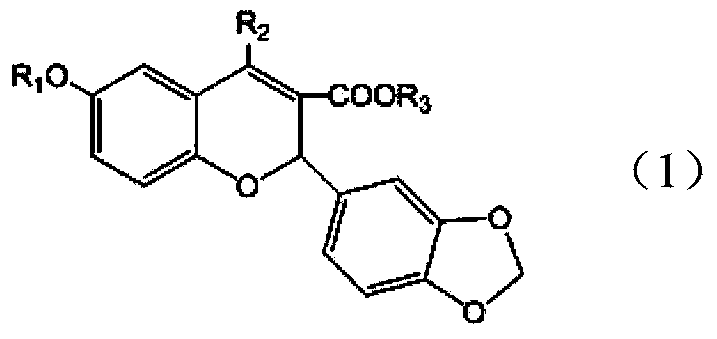
[0026] Example 1: Preparation of 2-(3,4-methylenedioxyphenyl)-4-chloro-6-isopropoxy-2H-chromene-3-carboxylic acid
[0027] The first step, prepare 2,5-dihydroxyacetophenone:
[0028]Add 110 g (1.0 mol) of hydroquinone, 206 g (2.02 mol) of acetic anhydride and 2 mL of concentrated sulfuric acid into a 1-liter beaker, stir the reaction mixture by hand, the reaction proceeds quickly and exotherms, continue to stir for ten minutes Finally, the reaction mixture was transferred to a 2-liter beaker containing 200 grams of crushed ice, stirred, and after the ice had completely melted, suction filtered, washed with water, and dried to obtain 170 grams of white solid powder, which was recrystallized with absolute ethanol to obtain White crystal hydroquinone diacetate 140 grams, yield 72%, Mp.120-122 ℃; To the 500mL round bottom flask equipped with the reflux condenser with calcium chloride drying tube, add 50 grams (0.257 mol) of hydroquinone diacetate and 116 grams (0.87mol) of powder...
Embodiment 2
[0037] Example 2: 2-(3,4-methylenedioxyphenyl)-4-[(4-methoxy)phenoxy]-6-isopropoxy-2H-chromene-3-carboxy acid preparation
[0038] The first step, prepare 2,5-dihydroxyacetophenone:
[0039] Operation with reference to the first step in Example 1;
[0040] The second step, preparation of 5-isopropoxy-2-hydroxyacetophenone:
[0041] Operation with reference to the second step in Example 1;
[0042] The third step is to prepare 2-(3,4-methylenedioxyphenyl)-6-isopropoxy-2,3-dihydro-4-chromone:
[0043] Operation with reference to the third step in Example 1;
[0044] The fourth step is to prepare 2-(3,4-methylenedioxyphenyl)-4-chloro-6-isopropoxy-2H-chromene-3-carboxylic acid:
[0045] Operation with reference to the fourth step in Example 1;
[0046] The fifth step is to prepare the target product:
[0047] Add 4 g (0.01 mol) of 2-(3,4-methylenedioxyphenyl)-4-chloro-6-isopropoxy-2H-chromene-3-carboxylic acid into a 250 mL Erlenmeyer flask , add 50mL of anhydrous DMF to d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com