Method for removing dibenzothiophene contained in fuel oil through catalytic oxidation
A technology of dibenzothiophene and catalytic oxidation, which is applied in the field of chemical engineering, can solve the problems of serious discharge pollution of waste lye, cannot be deeply oxidized, and has poor recyclability, and achieves good recyclability, low cost, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
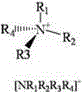
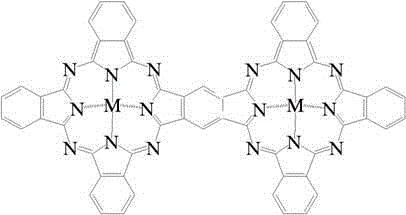
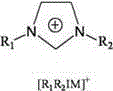
[0030] Weigh 5ml of n-octane model gasoline with a DBT concentration of 1000ppm, add 0.01g of binuclear metal phthalocyanine cobalt catalyst and 5ml of ionic liquid, in which the molar ratio of sulfur, catalyst and extractant is 1:0.0006:0.65, continuously in the reaction system Pass in oxidant air, control the air flow 0-100ml / min by the LZB-3WB glass rotor flowmeter, and react at 25℃ for 40min; after the reaction is over, stand still for stratification; pour out the upper oil phase for gas chromatography analysis, and The gas chromatography of the oil phase before the reaction showed that the desulfurization rate of DBT by binuclear metal phthalocyanine was 98.0%, and the catalyst was repeatedly used for 6 times, and the desulfurization rate was ≥94.0%.
Embodiment 2
[0032] Weigh 5ml of n-heptane model gasoline with a DBT concentration of 1000ppm, add 0.01g of binuclear metal phthalocyanine iron catalyst and 5ml of ionic liquid, in which the molar ratio of sulfur, catalyst and extractant is 1:0.0006:0.68, continuously in the reaction system Pass in oxidant air, control the air flow rate by LZB-3WB glass rotor flowmeter to 50ml / min, and react at 35℃ for 40min; after the reaction, let stand for stratification; pour out the upper oil phase for gas chromatography analysis, and The oil phase gas chromatographic comparison shows that the desulfurization rate of DBT by binuclear metal phthalocyanine is 97.5%, the catalyst is repeatedly used for 7 times, and the desulfurization rate is ≥93.7%.
Embodiment 3
[0034] Weigh 5ml of xylene model gasoline with a DBT concentration of 1000ppm, add 0.018g of binuclear metal phthalocyanine magnesium catalyst and 5ml of ionic liquid. The molar ratio of sulfur, catalyst and extractant is 1:0.0011:0.75. Into the oxidant air, the LZB-3WB glass rotor flowmeter controls the air flow to 50ml / min, and reacts at 40°C for 40min; after the reaction is over, stand still for stratification; pour out the upper oil phase for gas chromatography analysis, and the oil before the reaction Comparing with gas chromatography, the desulfurization rate of DBT by binuclear metal phthalocyanine is 97.33%. The catalyst is repeatedly used for 6 times, and the desulfurization rate is ≥93.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com