Preparation method of high-purity iopromide
A technology of iopromide and diamide, which is applied in the field of preparation of high-purity iopromide, can solve the problems of low reaction yield, long reaction route, difficult industrialized production and the like, and achieves the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
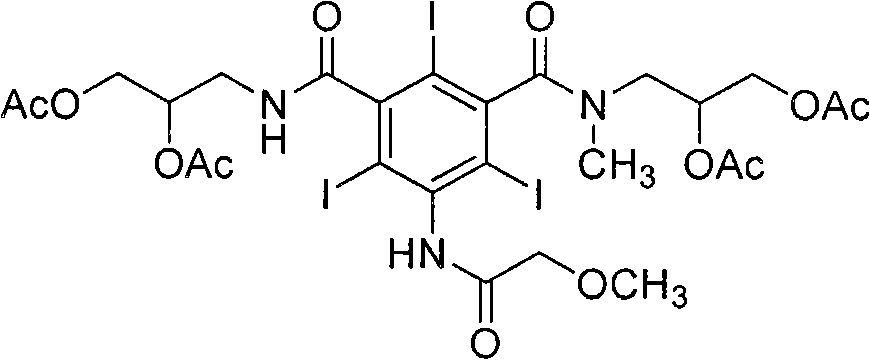
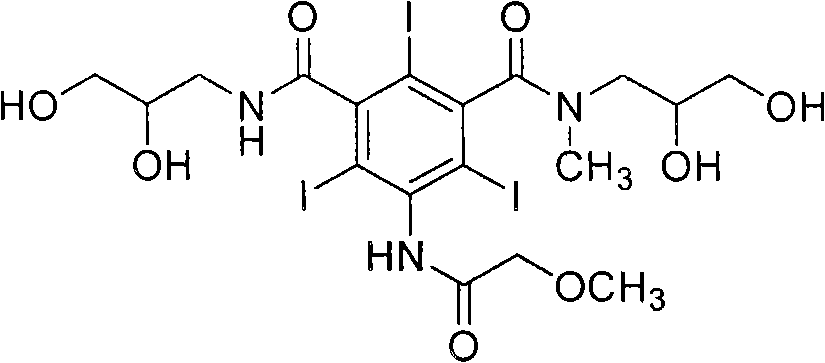
[0036] The preparation method of iopromide of formula (1) according to the present invention is shown in Reaction Scheme 5 below.
[0037] [Reaction Scheme 5]
[0038]
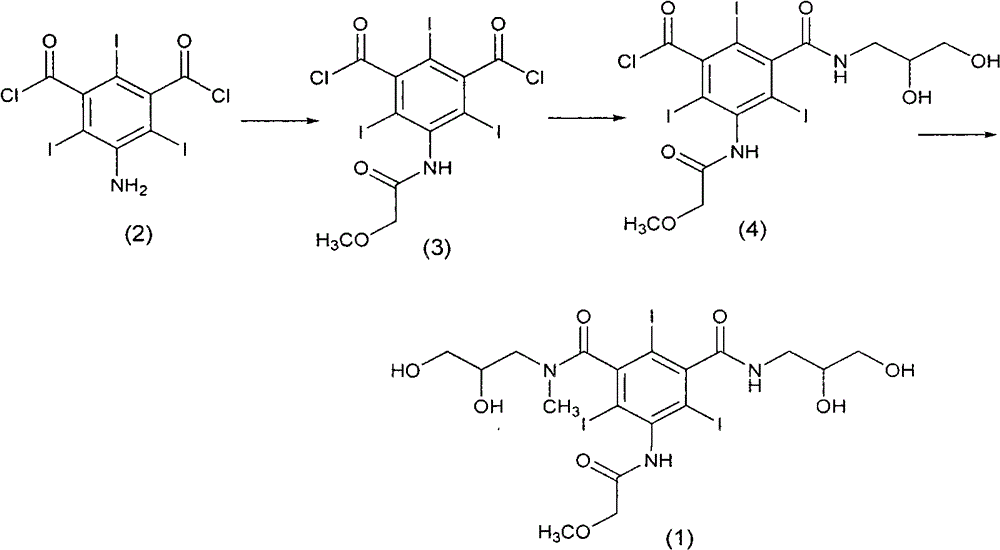
[0039] step 1
[0040] Wherein, in N, N dimethylformamide solvent, the compound of formula (2) is reacted with methoxyacetyl chloride to synthesize the compound of formula (3), and then in the presence of triethylamine, N, N Dimethylformamide is used as a solvent, and the compound of formula (3) is reacted with 2,3-dihydroxypropylamine to synthesize the compound of formula (4), as shown in Scheme 6.
[0041] [Reaction Scheme 6]
[0042]
[0043] In reaction scheme 6, the 2,3-dihydroxypropylamine used is preferably 0.7-1,0 equivalent, more preferably 0.8 equivalent, then can obtain the compound crude product of formula (1) in reasonable yield, make formula (1) The crude compound produces less by-products.
[0044] step 2
[0045] In the presence of the catalyst pyridine, using dichloromethane as a so...
Embodiment 1
[0052] Embodiment 1: the synthesis of 5-methoxyacetylamino-2,4,6-triiodophthaloyl chloride (formula 3)
[0053] Dissolve 5-amino-2,4,6-triiodo-1,3-phthaloyl chloride (200g, 0.34mol) in N,N-dimethylformamide (400mL), stir to dissolve, at room temperature , to which methoxyacetyl chloride (73.6g, 0.68mol) was added dropwise for 30min, then stirred at room temperature for 6 hours, detected by thin-layer chromatography, developer: methylene chloride: methanol = 5: 1 (V: V), raw material The main spots basically disappeared, and the reaction ended. The reaction solution was poured into ice water (2L), and a large amount of white solid was precipitated, then dichloromethane (1L) was added to dissolve, the water layer was separated, the organic phase was dried overnight with anhydrous magnesium sulfate, and the solvent was spin-off under reduced pressure to obtain a light yellow oil (203.6g, yield 92.8%).
Embodiment 2
[0054] Example 2: N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-5-[(2-methoxyacetyl)amino]-N'-methyl Synthesis of crude product of phenyl-1,3-carboxamide (formula 1)
[0055]With the US4364921 method, 5-methoxyacetamido-2,4,6-triiodo-1,3-phthaloyl chloride (203.6g, 0.31mol) was dissolved in N,N-dimethylformamide (250mL) , stirred to dissolve, the mixture was cooled to 0°C, and 3-amino-1,2-propanediol (22.9g, 0.25mol) in N,N-dimethylformamide (50mL) was slowly added dropwise thereto, and the addition was completed gradually After reaching room temperature, it was detected by thin-layer chromatography that the reaction of the raw materials was complete, and the developer: dichloromethane:methanol=5:1 (V:V), and the reaction was terminated. The reaction solution was added to a solution (3 L) of dichloromethane:petroleum ether=1:4 (V / V), and an oily substance gradually precipitated out. The supernatant was poured out and used directly in the next step. The oil was dissolved with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com