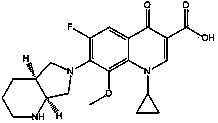
Preparation method of moxifloxacin intermediate
A technology for moxifloxacin and intermediates is applied in the field of preparation of moxifloxacin intermediates, which can solve the problems of highly toxic hydrogen fluoride smog, high equipment requirements, short reaction time, etc. Environmentally friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Synthesis of 6-benzyl-octahydro-pyrrolo[3,4-b]pyridine
[0028] Add 6-benzyl-hexahydro-pyrrolo[3,4-b]pyridine-5,7-dione (50g, 0.2mol) and 400ml tetrahydrofuran into a 3L three-necked flask and cool to 0-5°C , adding NaBH in batches 4 (37g, 1.0mol). After the addition is complete, slowly add 400 ml of concentrated sulfuric acid (49 g, 0.5 mol) in tetrahydrofuran dropwise at 0°C, controlling the internal temperature to not exceed 10°C. After dropping, the temperature was slowly raised to room temperature for 12 hours. Add 500ml of tetrahydrofuran and 500ml of water to dilute the reaction solution, add 49% sulfuric acid solution dropwise at 0°C, adjust the pH to 2, and reflux for 1h. Cool to 0°C, add solid sodium hydroxide to adjust the pH to 10, stir at room temperature for 30 min, extract with ethyl acetate three times, wash with saturated brine once, dry and concentrate to obtain 39 g of light yellow oily liquid with a yield of 88.3%. 1 H NMR (400MHz, CDC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com