Cyclohexane derivatives, preparation method thereof and applications thereof
A kind of technology of mixture and compound, applied in the field of cyclohexane derivative and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Compound of Example 1 Synthesis of (I-17)
[0150]
[0151] Step 1 (Ⅰ-17-a) Synthesis
[0152] Add 56.64g (0.24mol) 1,4-dibromobenzene (reactant) and 400ml dry tetrahydrofuran (solvent) to a 1L three-necked flask. ) n-butyllithium (reactant), keep it warm for 1 hour after dropping, at the same temperature, add dropwise a mixed solution of 0.216mol 3-methylcyclohexanone (reactant) and 50ml dry tetrahydrofuran (solvent), and stir for 30 minutes after dropping , naturally warming up, 200ml of saturated ammonium chloride aqueous solution was added dropwise at about 0°C (to adjust the pH value), the liquid was separated, the aqueous phase was extracted with 200ml of ethyl acetate (solvent), the organic phase was washed with water, and spin-dried to obtain 50g (GC: 89% ) liquid, add 50g of the product obtained above, 500ml of dry dichloromethane (solvent) into another 1L three-necked bottle, under nitrogen protection, cool down to -25°C, add dropwise 63.3ml (0.397mol,...
Embodiment 2
[0169] Synthesis of (Ⅰ-18)
[0170]
[0171] step 1 Synthesis of (I-18-a)
[0172] Add 75g (0.24mol) of 1,4-dibromobiphenyl (reactant) and 400ml of dry tetrahydrofuran (solvent) into a 1L three-necked flask. N) n-butyllithium (reactant), keep warm for 1 hour after dropping, at the same temperature, add dropwise a mixed solution of 0.216mol 3-methylcyclohexanone (reactant) and 50ml dry tetrahydrofuran (solvent), and stir for 30 Minutes, heat up naturally, add 200ml of saturated ammonium chloride aqueous solution dropwise at about 0°C (adjust the pH value), separate the liquid, extract the aqueous phase with 200ml of ethyl acetate (solvent), wash the organic phase with water, and spin dry to obtain 70g (GC: 89 %) liquid, add 70g of the product obtained above, 500ml of dry dichloromethane (solvent) to another 1L three-necked bottle, under nitrogen protection, cool down to -25°C, add dropwise 63.3ml (0.397mol, 2.2eq) triethyl Silicon hydrogen (reactant), after dropping, a...
Embodiment 3
[0194] Embodiment 3, preparation liquid crystal mixture a
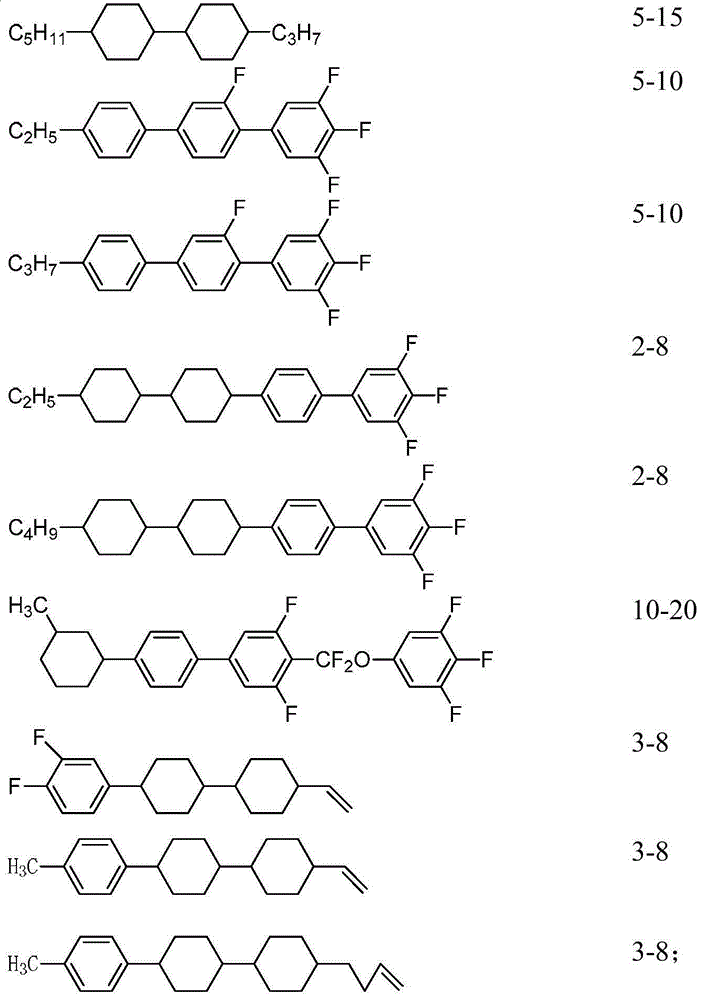
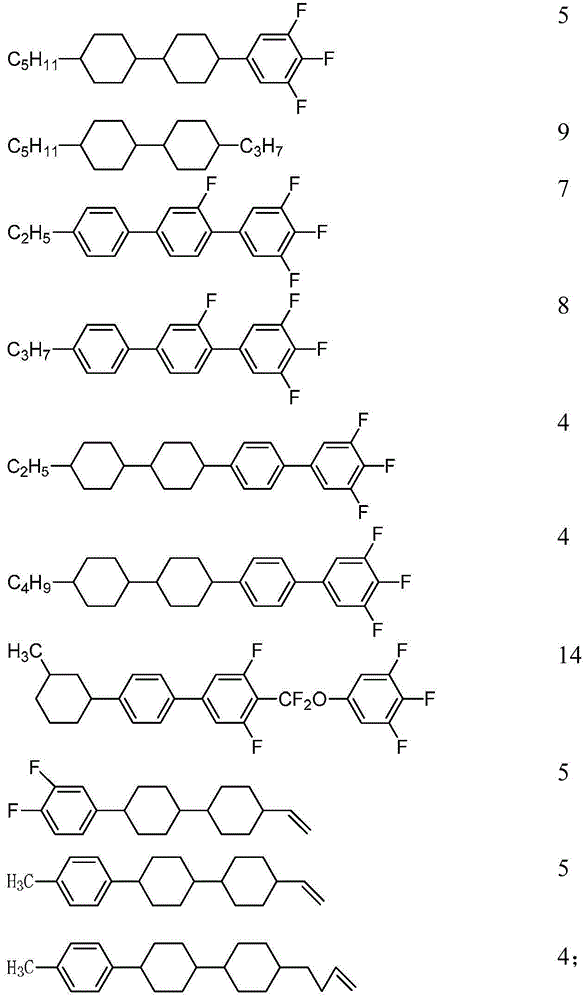
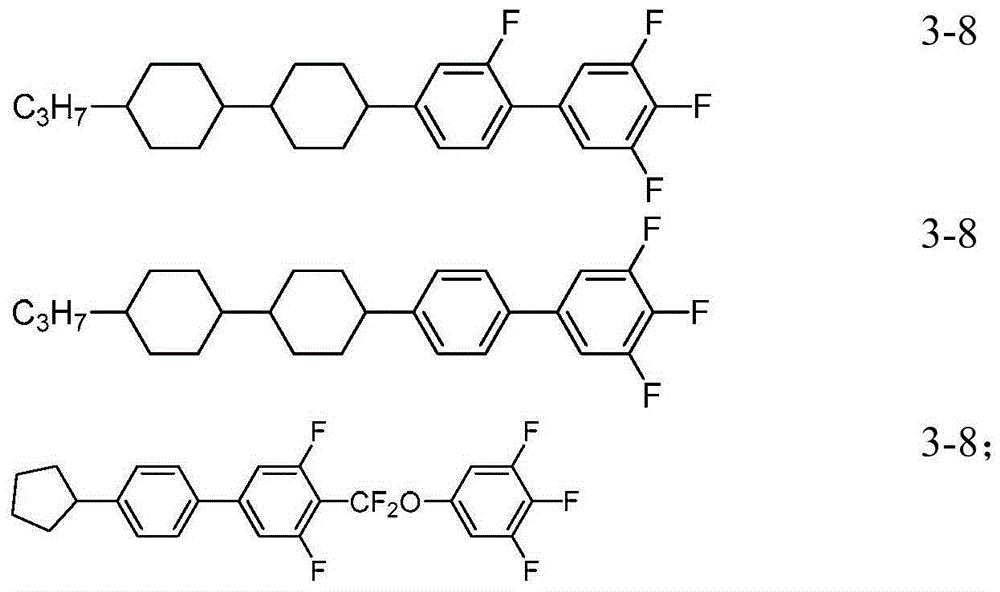
[0195] Mix the compounds uniformly according to the following mass percentages to obtain liquid crystal mixture a:
[0196]
[0197]
[0198] The performance test result of this liquid crystal mixture is as follows:
[0199] Cp: 88°C
[0200] Δn: 0.105
[0201] Δε: 7.3
[0202] gamma 1 :66;
[0203] It can be known from the above that the composition has a high clearing point, appropriate optical anisotropy, low rotational viscosity and fast response speed, and can be suitable for liquid crystal displays.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com