Al-18F mark fusion peptide and application thereof
A label fusion, al-18f technology, applied in the biological field, can solve the problems of pharmaceutical, inconvenient transportation, increased use cost, clinical application obstacles, etc., to improve probe concentration, improve specificity, and improve target/non-target The effect of target ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
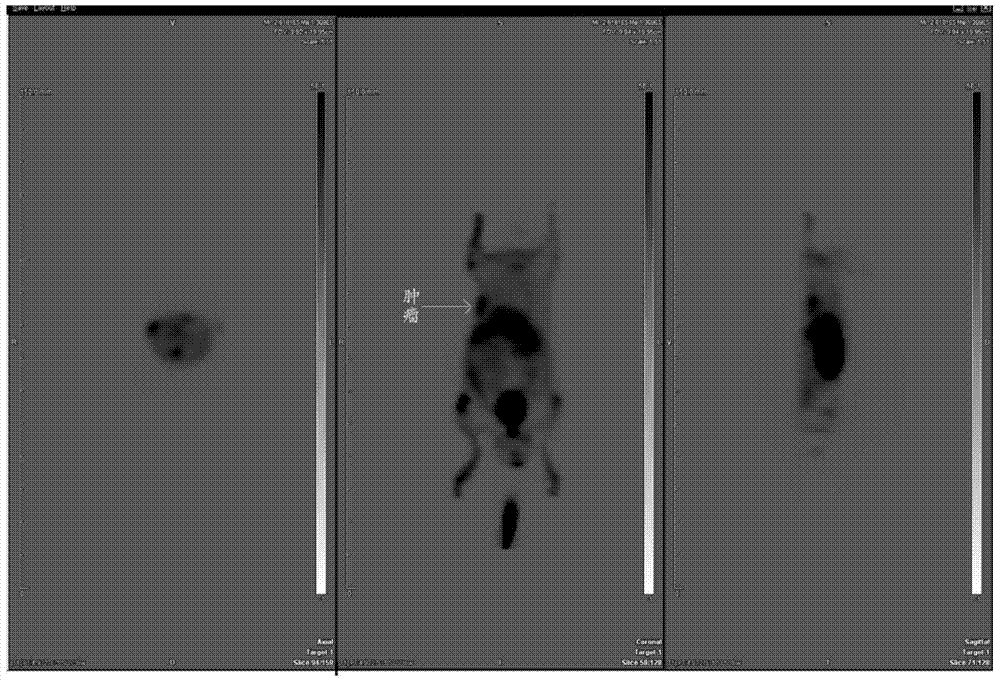
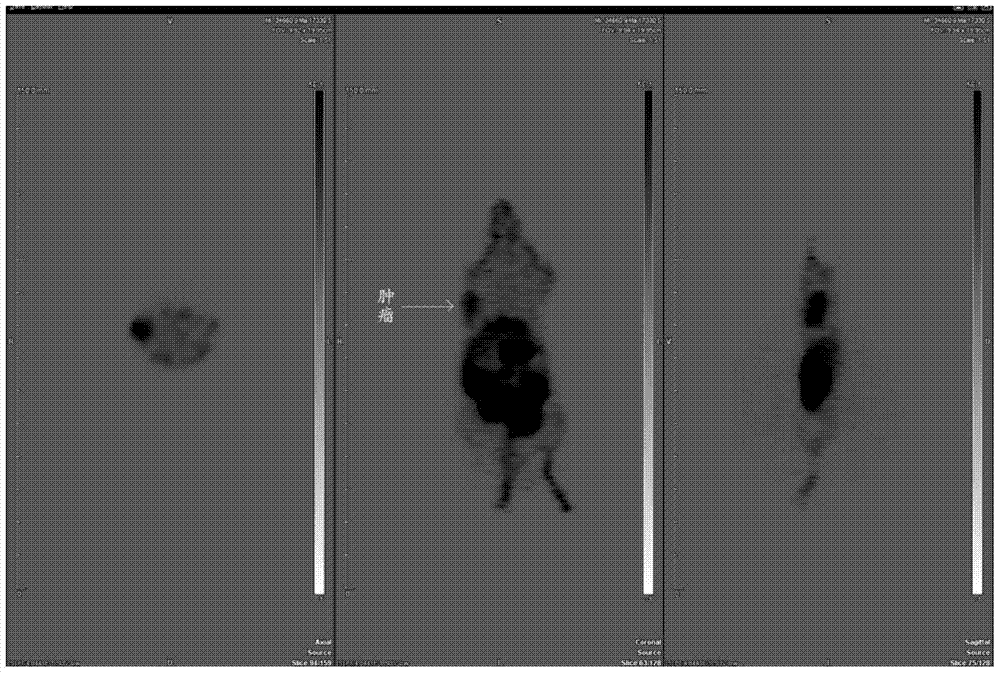
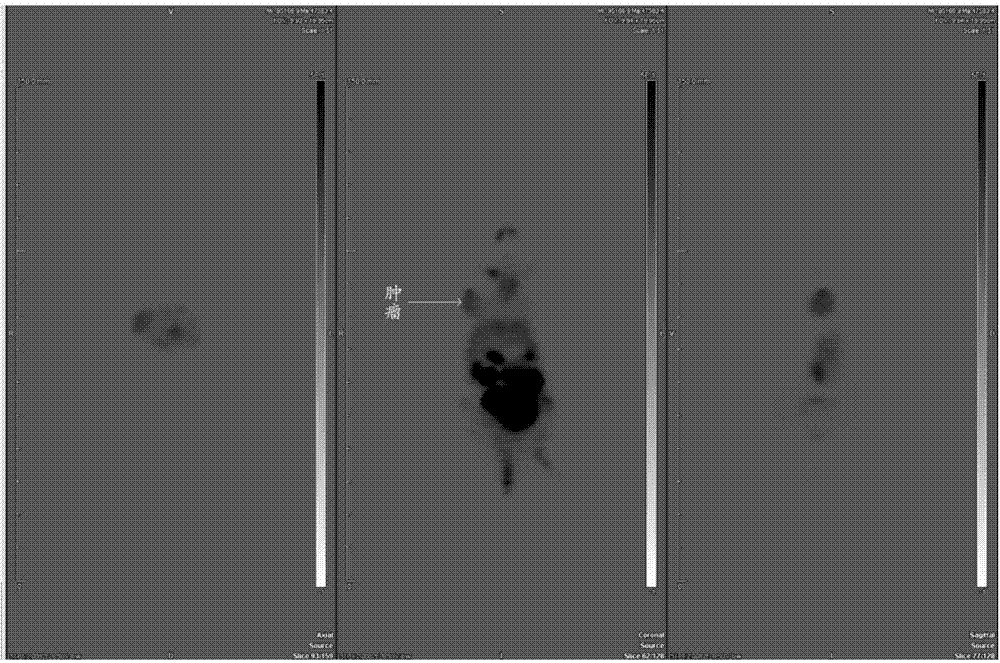
Image
Examples
Embodiment 1
[0036] (1) Preparation of NOTA-PEG-RGDyK-Lys-Ac-A7R:
[0037] The reaction formula is as follows:
[0038]
[0039] Specifically: RGDyK-Lys-Ac-A7R can be purchased from home and abroad (Hangzhou Zhongpei Biochemical Co., Ltd.). Dissolve [2-[2-(fluorenylmethoxycarbonyl-amino)ethoxy]ethoxy]acetic acid in 1 mL of DMF (dimethylformamide), add NHS (N-hydroxysuccinimide, also known as HOSU) and DCC (dicyclohexylcarbodiimide), stirred at room temperature for 2 hours. Add RGDyK-Lys-Ac-A7R to the above reaction solution, adjust the pH to 8.0-8.5 with DIEA (N,N-diisopropylethylamine), and stir overnight at room temperature. Add 3mL of 0.5M NH4OAc buffer solution (pH=7.0) to the reaction solution and filter, the filtrate is separated and purified by HPLC, the fractions of the target product are collected, combined and freeze-dried, the product fluorenylmethoxycarbonyl-PEG-RGDyK-Lys-Ac- Add 20% piperidine to A7R and react at room temperature for 30 minutes to remove the fluorenylmet...
Embodiment 2
[0048] This embodiment is a comparative example, and the contrast is [Al 18 F]NOTA-PEG-RGDyK, the structural formula is as follows:
[0049]
[0050] Its preparation method is as follows:
[0051] (1) RGDyK can be purchased from home and abroad. Dissolve [2-[2-(fluorenylmethoxycarbonyl-amino)ethoxy]ethoxy]acetic acid in 1 mL of DMF (dimethylformamide), add NHS (N-hydroxysuccinimide, also known as HOSU) and DCC (dicyclohexylcarbodiimide), stirred at room temperature for 2 hours. Add RGDyK to the above reaction solution, adjust the pH to 8.0-8.5 with DIEA (N,N-diisopropylethylamine), and stir overnight at room temperature. Add 3mL of 0.5M NH4OAc buffer solution (pH=7.0) to the reaction solution and filter, the filtrate is separated and purified by HPLC, the fractions of the target product are collected, combined and freeze-dried, the product fluorenylmethoxycarbonyl-PEG-RGDyK is added with 20% piperidine React at room temperature for 30 minutes to remove fluorenylmethoxyc...
Embodiment 3
[0057] This embodiment is a comparative example, and the contrast is [Al 18 F]NOTA-PEG3-A7R, the structural formula is as follows:
[0058]
[0059] (1) A7R can be purchased at home and abroad. Dissolve [2-[2-(fluorenylmethoxycarbonyl-amino)ethoxy]ethoxy]acetic acid in 1 mL of DMF (dimethylformamide), add NHS (N-hydroxysuccinimide, also known as HOSU) and DCC (dicyclohexylcarbodiimide), stirred at room temperature for 2 hours. Add ATWLPPR to the above reaction solution, adjust the pH to 8.0-8.5 with DIEA (N,N-diisopropylethylamine), and stir overnight at room temperature. Add 3mL of 0.5M NH4OAc buffer solution (pH=7.0) to the reaction solution and filter, the filtrate is separated and purified by HPLC, the fractions of the target product are collected, combined and freeze-dried, the product fluorenylmethoxycarbonyl-PEG-A7R is added with 20% piperidine React at room temperature for 30 minutes to remove fluorenylmethoxycarbonyl, then add 2,2'-(7-(2-((2,5-dioxopyrrolidin-1-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com