Method for resolving DL-menthol through catalysis of lipase
A technology of menthol and lipase, applied in the field of preparing L-menthol and splitting DL-menthol, can solve problems such as complicated steps, difficult separation of acylating agent and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
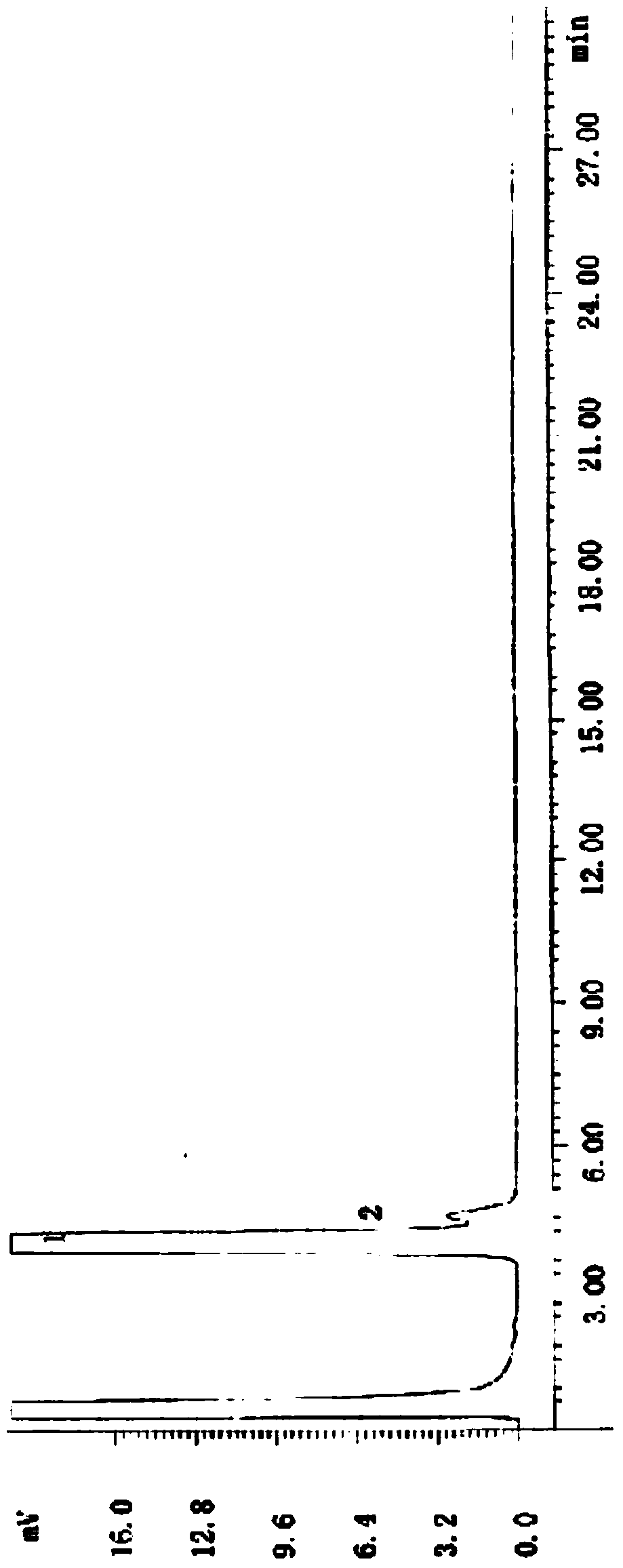
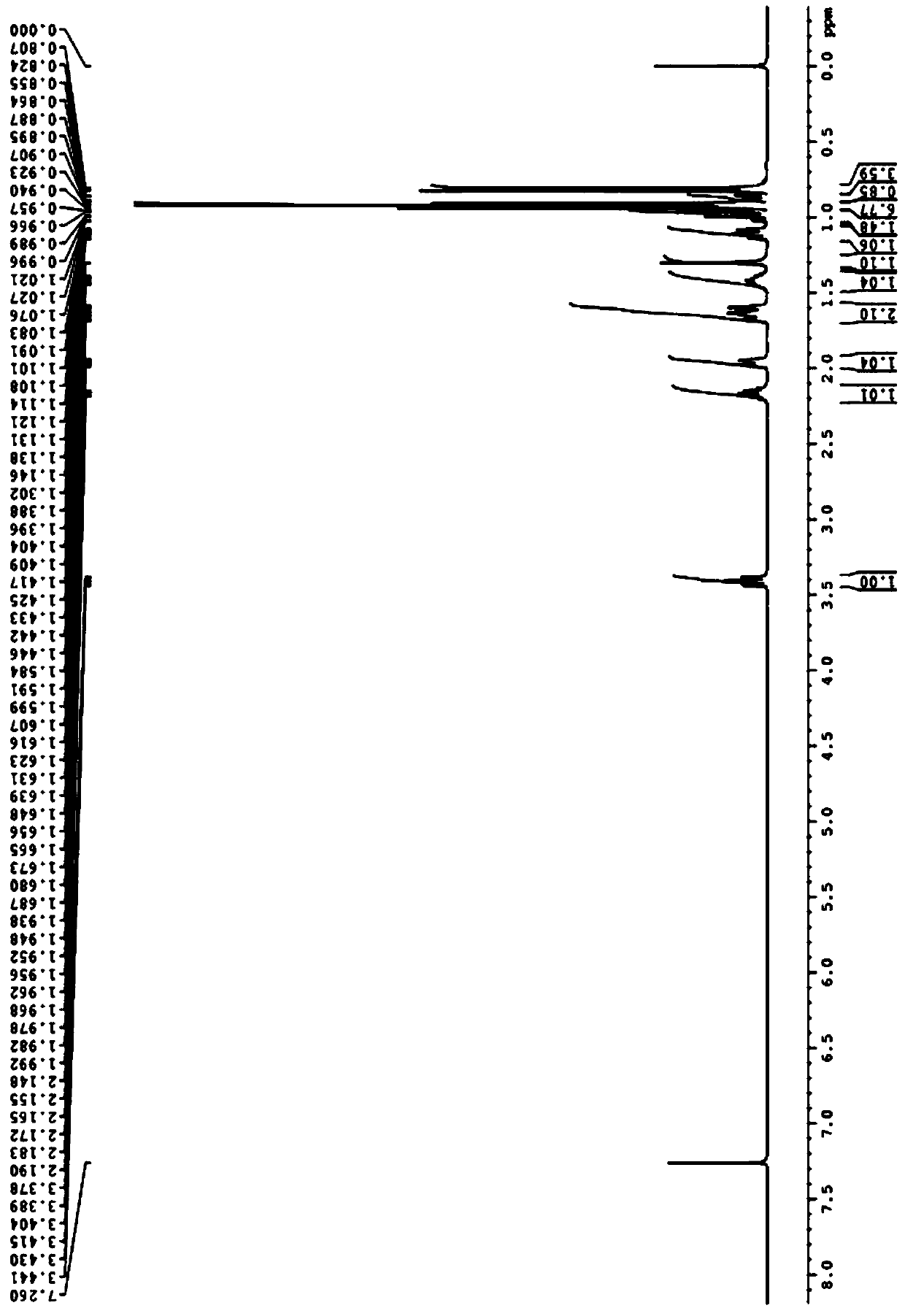
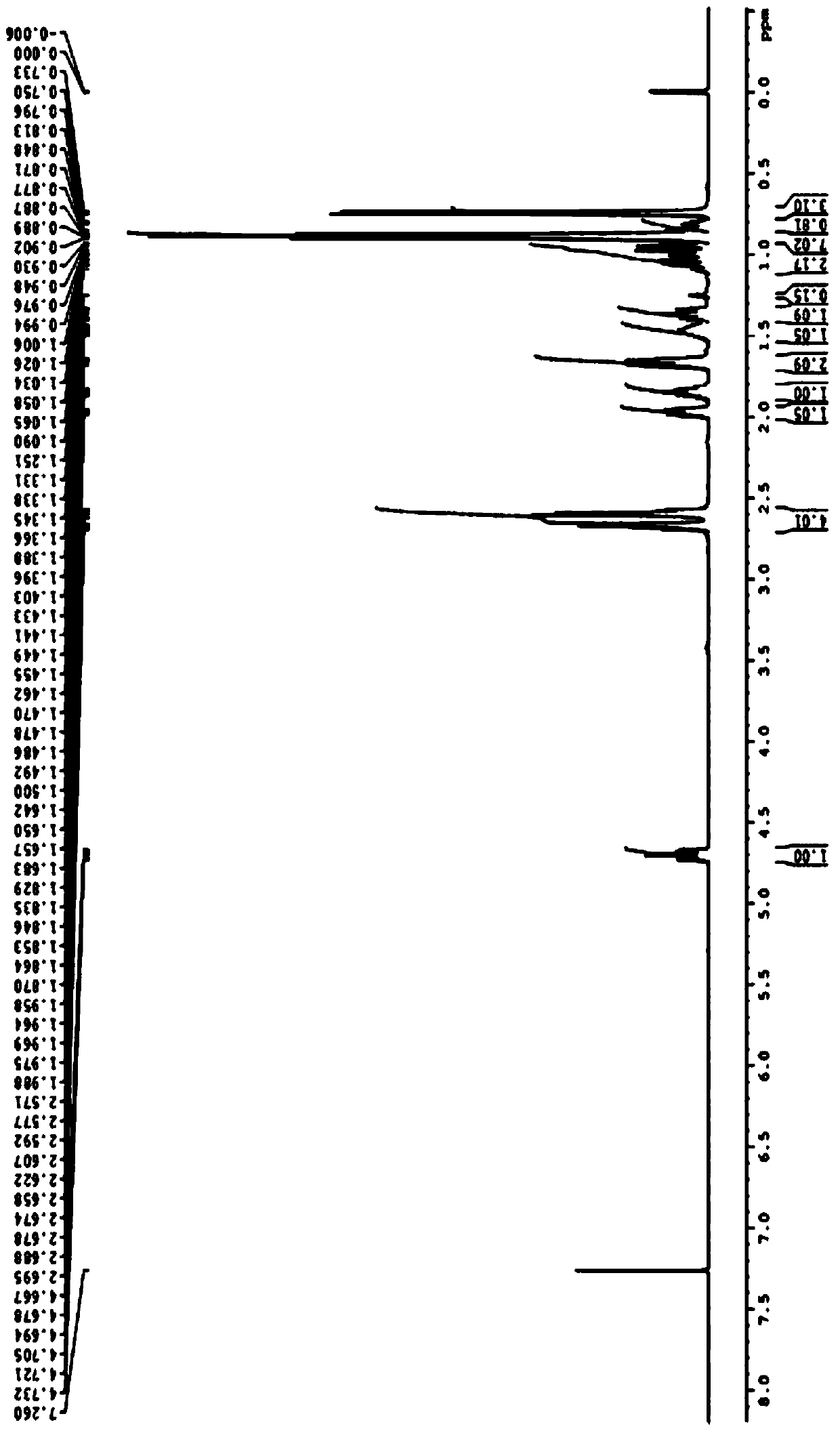
[0025] Specific embodiment 1: In this embodiment, the lipase-catalyzed splitting method of DL-menthol is completed through the following steps: Step 1, 5mmolDL-menthol, 1777U / mmol porcine pancreatic lipase (PPL), 30mmol succinic anhydride Mix it with 50mL ether and add it to a 250ml Erlenmeyer flask, then shake it on a shaker at 30°C at 200r / min for 48h, filter to remove porcine pancreatic lipase, dilute with 20mL ether, and then add 20mL NaCO with a molar concentration of 1mol / L 3 solution, and then extracted three times with ether, the amount of ether extracted each time was 20mL, with MgSO 4 After drying, diethyl ether is removed by rotary evaporation under reduced pressure to obtain D-menthol; the enantiomeric excess rate of D-menthol can reach 95ee%, and the conversion rate can reach 87% (the calculation formula is as follows).
[0026] Step 2. Stir and hydrolyze the remaining solution after step 1 with NaOH solution at room temperature for 5 hours, then extract with ethe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com