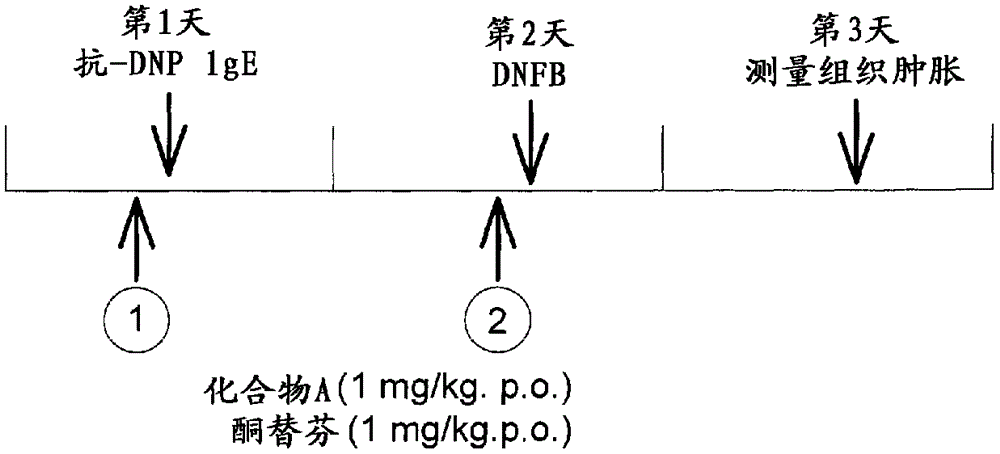
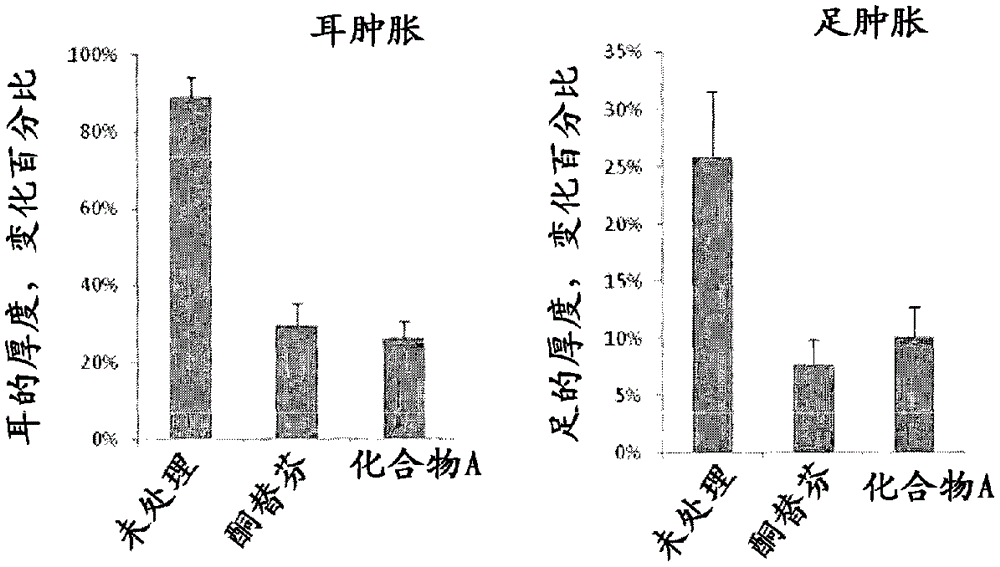
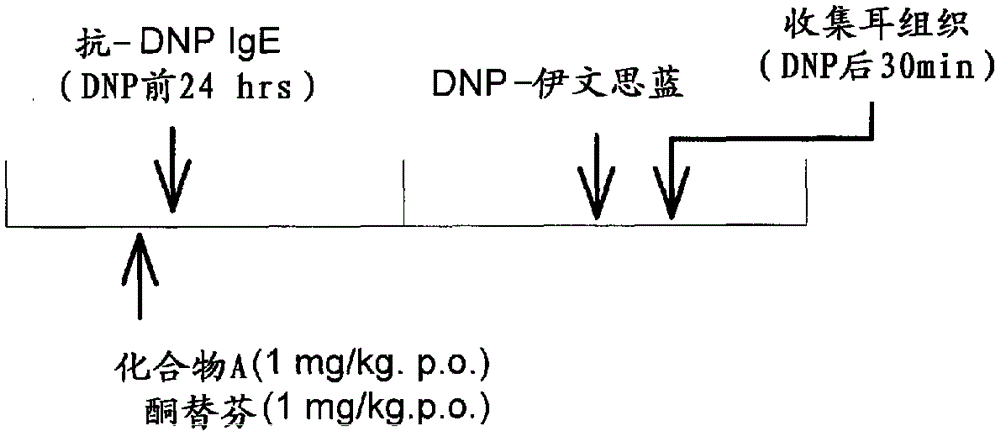
Anti-allergic benzocycloheptathiophene derivatives
An allergy and benzocycloheptane technology, applied in the direction of active ingredients of heterocyclic compounds, drug combinations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0277] Example 1. Synthesis of 4-[1-(5-methyl-pyridin-3-ylmethyl)piperidin-4-ylidene]-4,9-dihydro-1-thia-benzo[f] Azulene-10-one.
[0278]
[0279] Scheme I gives 4-[1-(5-methyl-pyridin-3-ylmethyl)piperidin-4-ylidene]-4,9-dihydro-1-thia-benzo[f] Azulene-10-one ( 5 ), which passes through norketotifen ( 2 ) and 3-bromomethyl-5-methyl-pyridine ( 4 ) Alkylation. By treating 3,5-lutidine with NBS and AIBN in a solvent mixture of chloroform and carbon tetrachloride ( 3 ) to obtain the compound ( 4 ). Norketotifen ( 2 ) was obtained by treating ketotifen with 1-chloroethyl chloroformate and methanol, respectively. Norketotifen ( 2 ) can also be obtained by treating ketotifen with vinyl chloroformate. Similarly, norketotifen ( 2 ) can be obtained by treating ketotifen with 2,2,2-trichloroethyl chloroformate (see US Patent 7,557,128). Alternatively, norketotifen ( 2 ) can be obtained by treating ketotifen with cyanogen bromide (von Braun dealkylation) or ethyl chlorofo...
Embodiment 2
[0280] Example 2. Synthesis of 4-[1-(5-methyl-pyridin-3-ylmethyl)piperidin-4-ylidene]-4,9-dihydro-1-thia-benzo[f] Azulene-10-one. (Synthetic Scheme II)
[0281]
[0282] In protocol II, norketotifen ( 2 ) and 5-methyl-nicotinic acid ( 6 ) acylation to generate compound (7), and then available phosphorus oxychloride and sodium borohydride reduction can obtain 4-[1-(5-methyl-pyridin-3-ylmethyl)piperidin-4-ylidene]- 4,9-dihydro-1-thia-benzo[f]azulene-10-one ( 5 ) (please refer to Journal of Medicinal Chemistry 1994, 37, 2697-2703).
Embodiment 3
[0283] Example 3. Synthesis of 4-[1-(5-methyl-pyridin-3-ylmethyl)piperidin-4-ylidene]-4,9-dihydro-1-thia-benzo[f] Azulene-10-one. (Synthetic Scheme III)
[0284]
[0285]
[0286] In Scheme III, the compound ( 8 ) and Grignard derivatives prepared from 3-(4-chloro-piperidin-1-ylmethyl)-5-methyl-pyridine (references: DrugsoftheFuture1996, 21(10):1032-1036 and SpanishPatentES2120899) ( The reaction of 9) provides the alcohol ( 10 ), the alcohol ( 10 ) through H 2 SO 4 Final elimination of HBr and dehydration to yield 4-[1-(5-methyl-pyridin-3-ylmethyl)piperidin-4-ylidene]-4,9-dihydro-1-thia-benzo[ f] azulene-10-one ( 5 ). Alternatively, alcohol ( 10 ) is dehydrated by HBr to generate the compound ( 11 ), the compound ( 11 ) was treated with piperidine and potassium tert-butoxide to provide the compound ( 12 ). compound ( 12 ) is treated with aqueous hydrochloric acid to generate 4-[1-(5-methyl-pyridin-3-ylmethyl)piperidin-4-ylidene]-4,9-dihydro-1-thia-benzo[f ]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com