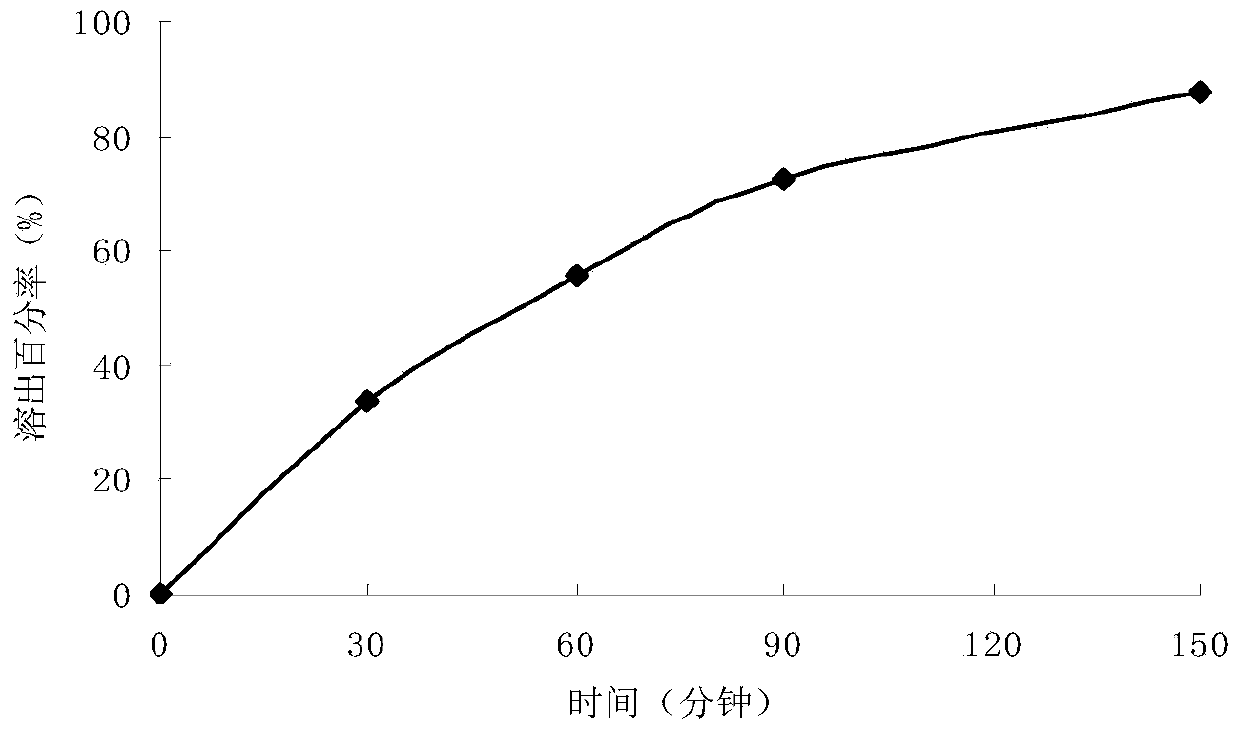
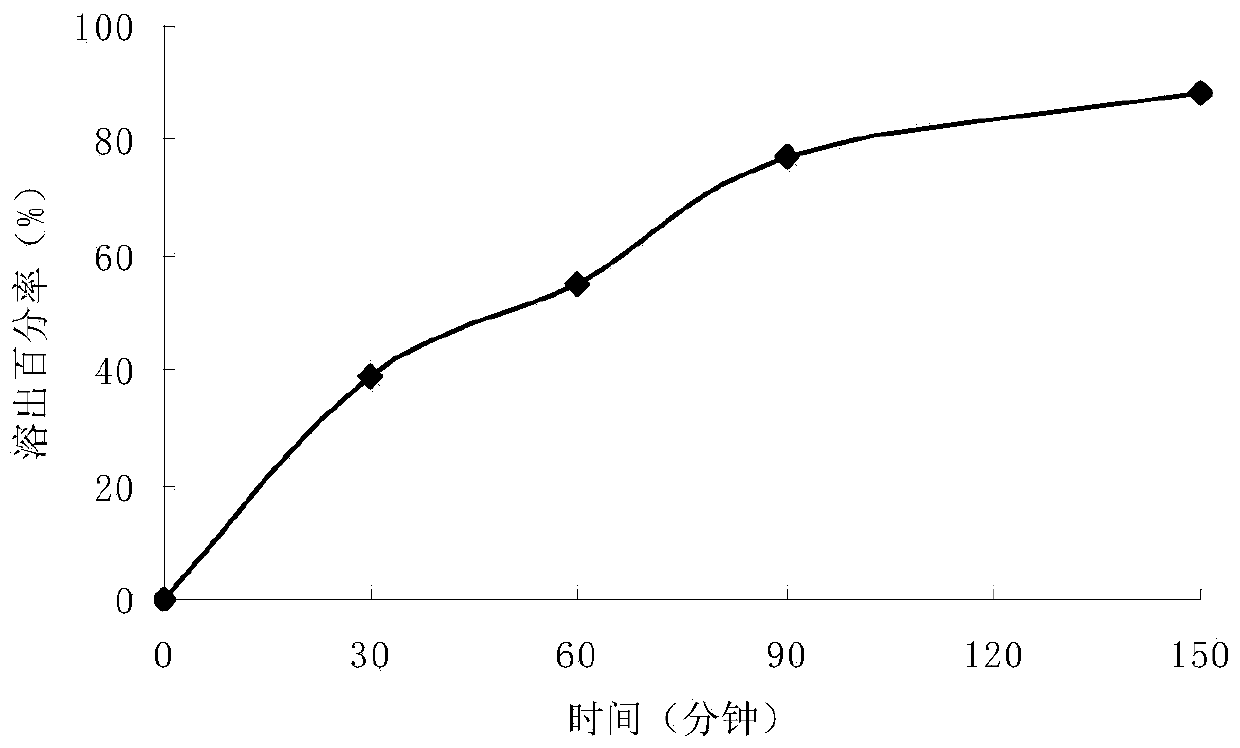
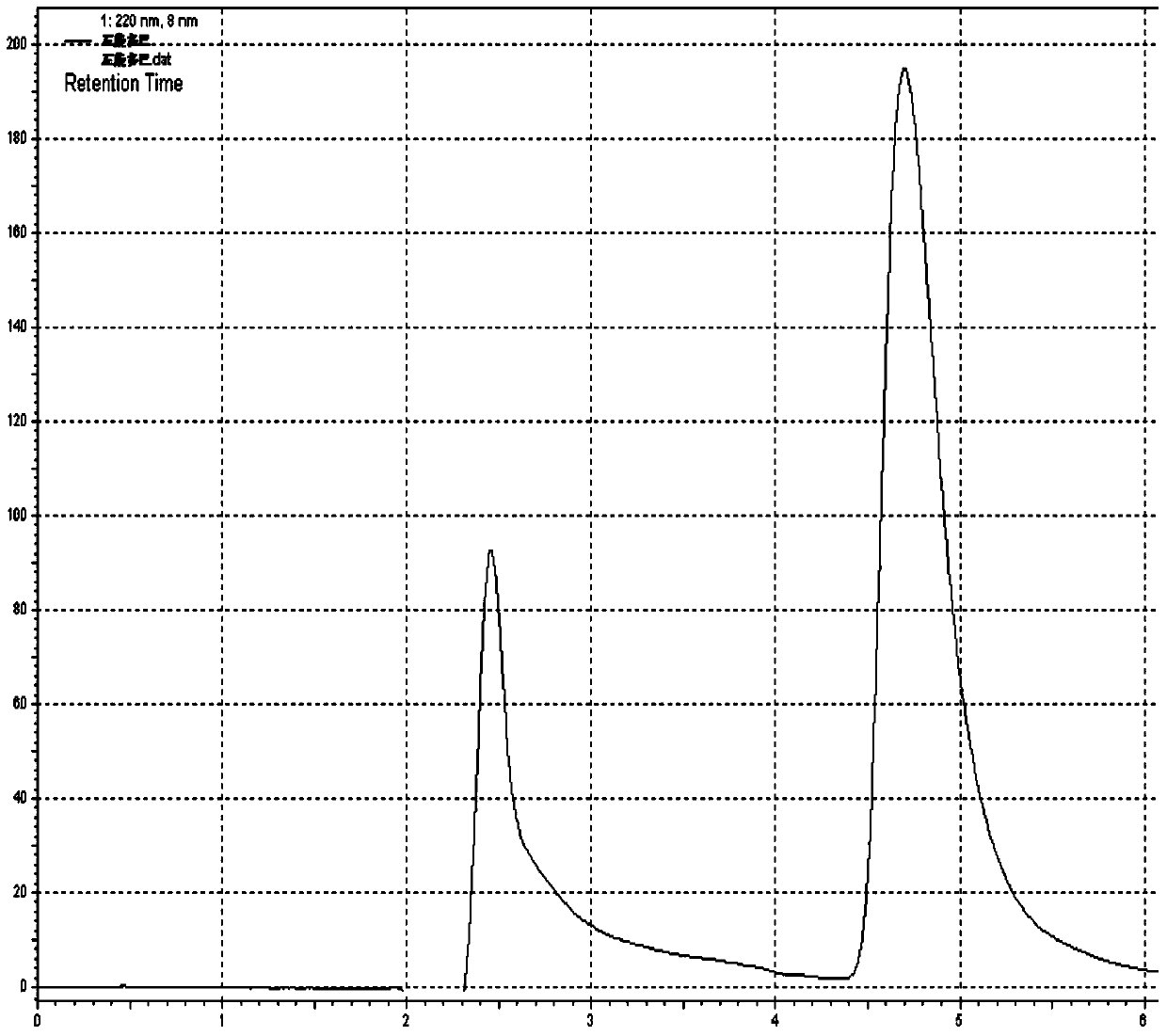
Levodopa/carbidopa compound sustained-release suspension and preparation method thereof
A technology of levodopa and suspension preparations, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as dysphagia, poor swallowing ability, and poor drug stability. Achieve the effects of good reproducibility and consistency, convenient administration, and increased plasticity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] 1. Preparation of Drug Resin
[0034] The cation exchange resin is added into the levodopa solution and stirred at room temperature. Samples were taken regularly to determine the concentration of the drug in the solution. When the drug concentration no longer changes with time, the equilibrium is reached, the unbound drug on the surface of the resin is washed away with double distilled water, and the drug-loaded resin is obtained by drying at 40°C-60°C.
[0035] Add the cation exchange resin to the carbidopa solution and stir at room temperature. Samples were taken regularly to determine the concentration of the drug in the solution. When the drug concentration no longer changes with time, the equilibrium is reached, the unbound drug on the surface of the resin is washed away with double distilled water, and the drug-loaded resin is obtained by drying at 40°C-60°C.
[0036] 2. Impregnation of drug resin
[0037] Take an appropriate amount of drug-loaded resin, add i...
Embodiment 1
[0046] 1. Preparation of Drug Resin
[0047]Take 200 mg of ion exchange resin and add 200 ml of 1.0 mg / ml levodopa solution (dissolved in 0.001 mol / L HCl solution), and stir at room temperature for 2 hours. Wash the unbound drug on the surface of the resin with double distilled water, and dry at 60°C to obtain the drug-loaded resin.
[0048] Take 200 mg of ion exchange resin and add 200 ml of 1.0 mg / ml carbidopa solution (dissolved in 0.001 mol / L HCl solution), and stir at room temperature for 2 hours. Wash the unbound drug on the surface of the resin with double distilled water, and dry at 60°C to obtain the drug-loaded resin.
[0049] 2. Impregnation of drug resin
[0050] Take 100 mg of drug-loaded resin, add 10 ml of 20% PEG6000 solution, stir for 0.5 hour, dry and sieve to obtain impregnated drug resin.
[0051] 3. Preparation of Drug Resin Microcapsules
[0052] Dissolve 40mg of acrylic resin in 8ml of acetone, then add 200mg of impregnated drug-loaded resin and 1ml ...
Embodiment 2
[0056] 1. Preparation of Drug Resin
[0057] Take 200 mg of ion exchange resin and add 200 ml of 5.0 mg / ml levodopa solution (dissolved in 0.001 mol / L HCl solution), and stir at room temperature for 2 hours. Wash the unbound drug on the surface of the resin with double distilled water, and dry at 60°C to obtain the drug-loaded resin.
[0058] Take 200 mg of ion exchange resin and add 200 ml of 5.0 mg / ml carbidopa solution (dissolved in 0.001 mol / L HCl solution), and stir at room temperature for 2 hours. Wash the unbound drug on the surface of the resin with double distilled water, and dry at 60°C to obtain the drug-loaded resin.
[0059] 2. Impregnation of drug resin
[0060] Take 100 mg of drug-loaded resin, add 10 ml of 20% PEG6000 solution, stir for 0.5 hour, dry and sieve to obtain impregnated drug resin.
[0061] 3. Preparation of Drug Resin Microcapsules
[0062] Dissolve 60mg of acrylic resin in 8ml of acetone, then add 200mg of impregnated drug-loaded resin and 1ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com