Effects of galectin-9 on systemic lupus erythematosus or similar inflammatory diseases
A galectin, systemic technology, applied in the field of biomedicine, can solve the problem of no research report on the effect of galectin-9, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Expression of recombinant human galectin-9 (Gal-9) protein:
[0062] Through the method of chemical synthesis, design and synthesize the sequence of recombinant human Gal-9 protein gene (SEQ ID No: 2), the sequence includes length Ala2-Thr323 total 322AA, (NCBI Accession#BAA31542), the front end is added to the expression vector Not I restriction site, 6 His (used for affinity chromatography, combined with nickel column to reduce purification steps) and EK restriction site, including stop codon TAA, and KpNI restriction enzyme for cloning The linker is 9bp in total.
[0063] The recombinant human Gal-9 protein gene monomer was excised with restriction endonucleases Not I and KpNI, connected to the vector pET32a digested with Not I and KpN I, transformed into E. coli, and screened for ampicillin-resistant transformants child. After plasmid extraction, restriction enzyme digestion identification proved that the recombinant human Gal-9 protein gene monomer has been ...
Embodiment 2
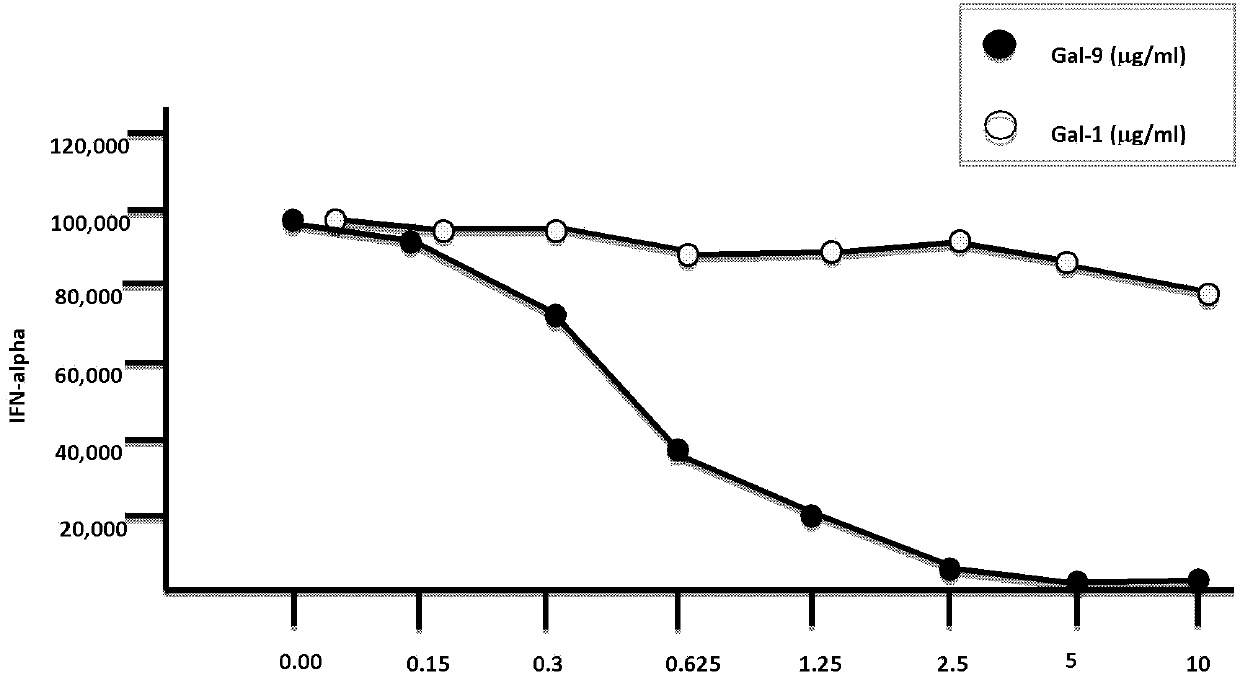
[0066] Example 2 Cultivation of human plasmacytoid dendritic cells and the study of affecting the secretion of type I interferon in plasmacytoid dendritic cells
[0067] Materials and Method:
[0068] R848 (1μg / mL) (purchased from InvivoGen, San Diego, CA)
[0069] CpG typeA oligodeoxynucleotide chain (2216, 5μM), type B (2006, 5μM), type C (C274, 5μM)
[0070] γ-irradiated herpes simplex virus (HSV)-1 (KOS strain; 10PFU / cell)
[0071] Influenza virus (PR8strain; 10PFU / cell)
[0072] Sendai virus (Cantellstrain; 5PFU / cell)
[0073] Recombinant Galectin-1 (purchased from R&D Systems, Minneapolis, MN)
[0074] Recombinant human GM-CSF (100ng / mL, purchased from R&D Systems, Minneapolis, MN)
[0075] Recombinant human IL-4 (200ng / mL, purchased from R&D Systems, Minneapolis, MN)
[0076] IFN-α ELISA kit (PBL, Biomedical Laboratories, Piscataway, NJ)
[0077] Human CD14 beads (purchased from Miltenyi Biotec, Auburn, CA)
[0078] BDCA4+ separation kit (purchased from Miltenyi Biotec, Auburn, CA)
[00...
Embodiment 3
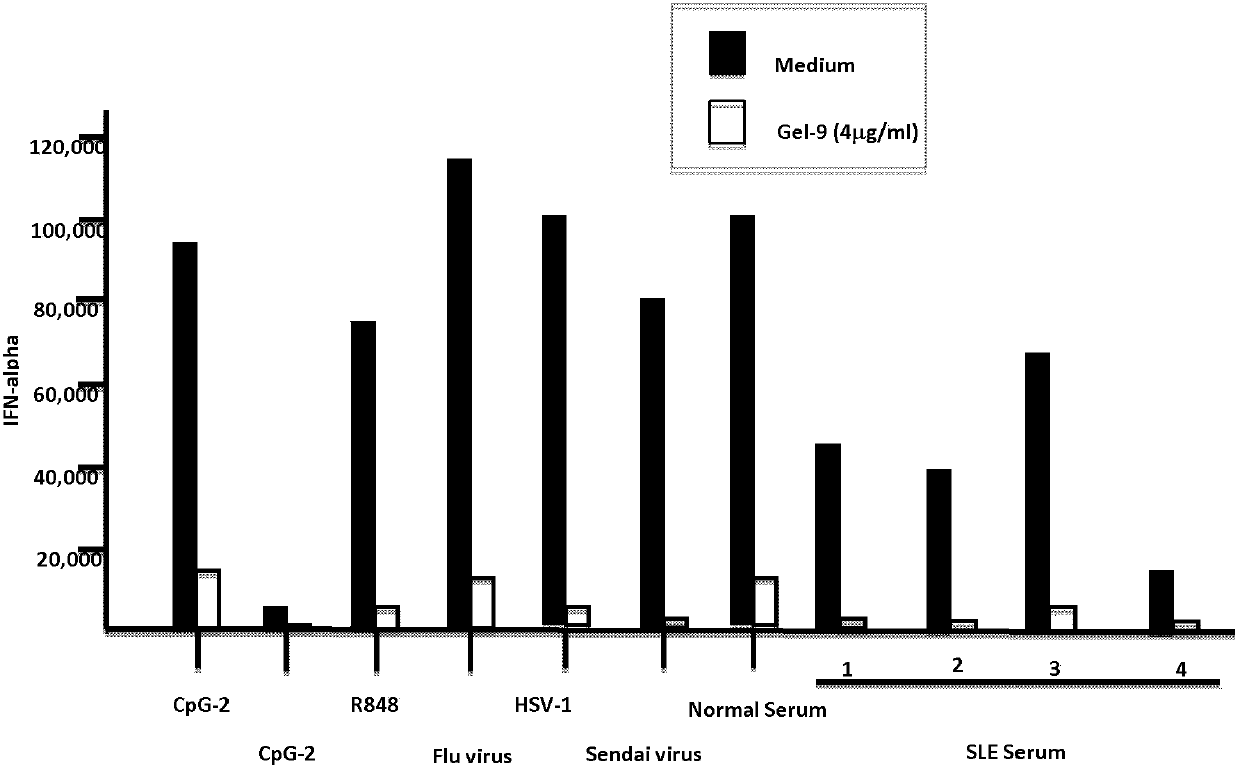
[0091] Example 3 Study on the inhibitory effect of galectin-9 on the secretion of type I interferon in plasmacytoid dendritic cells
[0092] Materials and Method:
[0093] R848 (1μg / mL) (purchased from InvivoGen, San Diego, CA)
[0094] CpG typeA oligodeoxynucleotide chain (2216, 5μM), type B (2006, 5μM), type C (C274, 5μM)
[0095] γ-irradiated herpes simplex virus (HSV)-1 (KOS strain; 10PFU / cell)
[0096] Influenza virus (PR8strain; 10PFU / cell)
[0097] Sendai virus (Cantellstrain; 5PFU / cell)
[0098] Reorganization of Galectin-1 (R&D Systems, Minneapolis, MN)
[0099] Recombinant human GM-CSF (100ng / mL, purchased from R&D Systems, Minneapolis, MN)
[0100] Recombinant human IL-4 (200ng / mL, purchased from R&D Systems, Minneapolis, MN)
[0101] IFN-α ELISA kit (PBL, Biomedical Laboratories, Piscataway, NJ)
[0102] Human CD14 beads (purchased from Miltenyi Biotec, Auburn, CA)
[0103] BDCA4+ separation kit (purchased from Miltenyi Biotec, Auburn, CA)
[0104] Anti-human CD123-PE, anti-human CD...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com