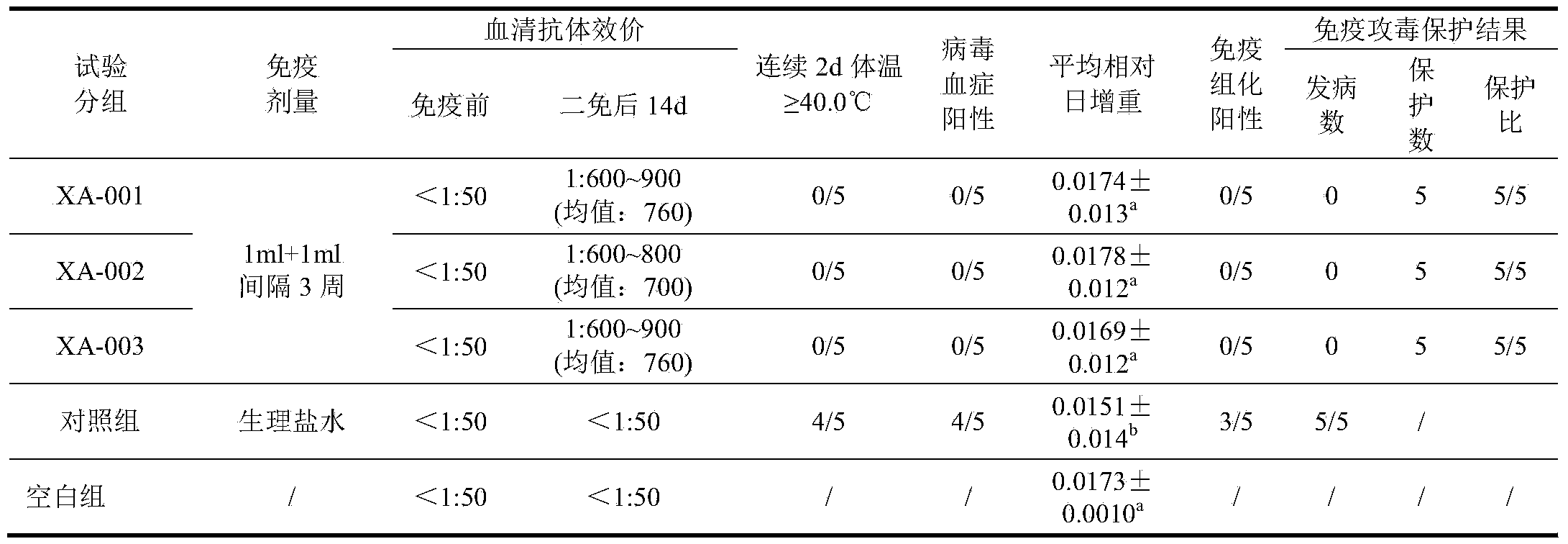
Preparation method of porcine circovirus type 2 inactivated vaccine
A porcine circovirus and inactivated vaccine technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, antiviral agents, etc., can solve the problems of low safety and large side effects, and achieve high safety, small side effects, The effect of high product quality controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
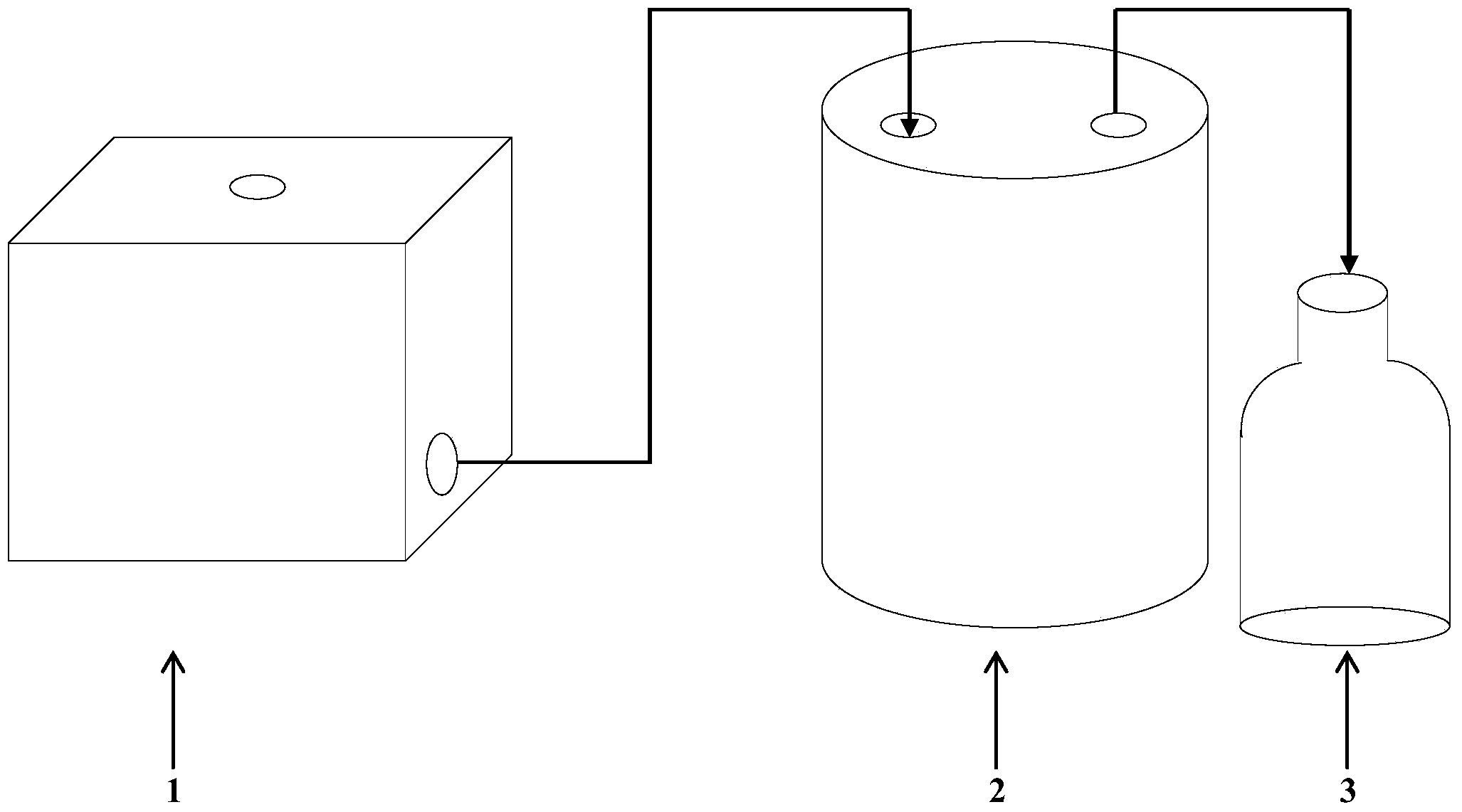
[0026] This example illustrates the determination of PK-15 cell culture circovirus bioreactor culture conditions and PCV-2 proliferation conditions.
[0027] (1) Put 2×10 5 PK-15 cells per ml were inserted into the reactor, supplemented with DMEM medium containing 5% bovine serum to 5L, set the stirring speed at 90 rpm, dissolved oxygen at 50%, pH 7.2, and temperature 37 ℃, carry out cell suspension culture, turn on the inlet pump and outlet pump for fed-batch culture after 24 hours, take regular samples every day to detect the consumption of glucose and record the consumption of lye (1%NaOH), and continue to cultivate for 13 days. Open the jar, digest and count the cells. The final culture conditions were determined as follows: 90 rpm, dissolved oxygen 50%, pH 7.2, temperature 37°C.
[0028] (2) When the cell reactor is cultivated for 48 hours, 72 hours and 96 hours, the cell growth liquid is discharged, the maintenance liquid containing 1% serum is inserted into the reacto...
Embodiment 2
[0030] This example illustrates the method of inoculating and culturing the virus to prepare the virus solution.
[0031] The volume is 1L and the concentration is 2~3×10 5 PK-15 cells per ml were inserted into the reactor, supplemented with DMEM medium containing 5% bovine serum to 5L, set the stirring speed at 90 rpm, dissolved oxygen at 50%, pH 7.2, and temperature 37 ℃, carry out cell suspension culture, after 24 hours, turn on the liquid inlet pump and the liquid outlet pump for fed-batch culture; after the cell reactor is cultivated for 72 hours, the cell growth liquid is discharged, and the maintenance liquid containing 1% serum is inserted into the reactor , insert the prepared circovirus (YZ strain) F15 seed virus, the inoculation volume is 400mL, add D-glucosamine with a final concentration of 2mmol / L to the virus maintenance solution, adjust the pH to 7.4, and set the temperature At 36.5°C, harvest the venom after 24 hours of cultivation and replenish fresh mainten...
Embodiment 3
[0033] This example illustrates the method of inactivating virus liquid and preparing vaccine products.
[0034] Inactivation of virus fluid:
[0035]The virus liquid finally obtained in Example 2 was collected and inactivated with cyclized BEI with a final concentration of 1.0‰ at a central temperature of 32°C for 48 hours, and then terminated with sterile sodium thiosulfate solution with a final concentration of 2%.
[0036] Preparation of inactivated vaccine:
[0037] Take 1 part of MontanideTM ISA206VG adjuvant from Sepic Company in France, 1 part of qualified inactivated antigen, mix them in an equal amount in an emulsification tank, stir and emulsify at a low speed for 30 minutes, and prepare PCV-2 (YZ strain) water-in-oil-in-water Dosage forms of inactivated vaccines.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com