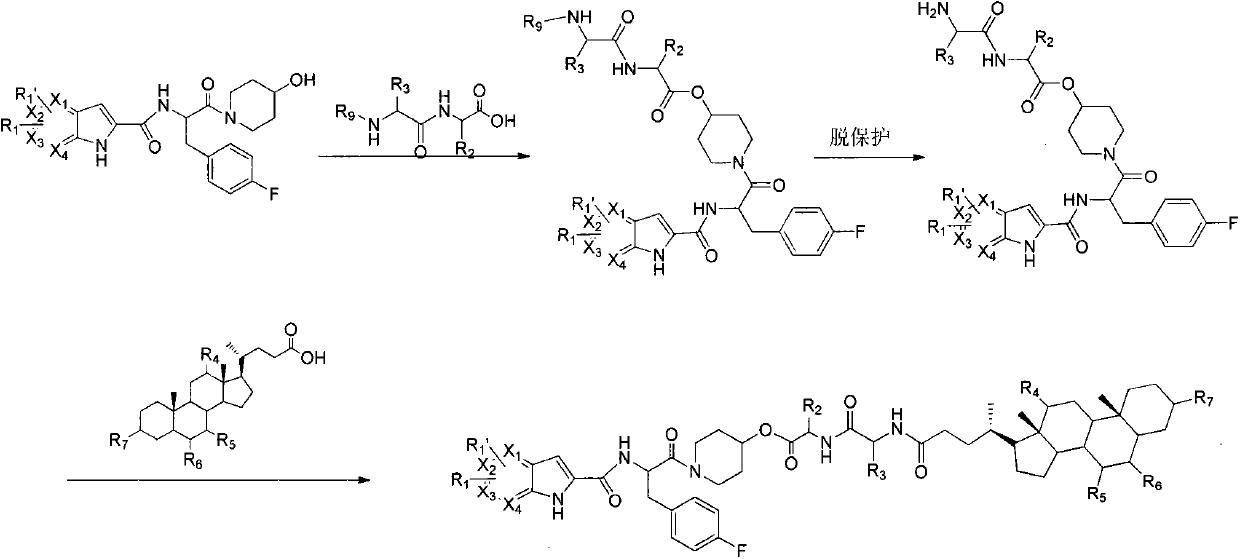
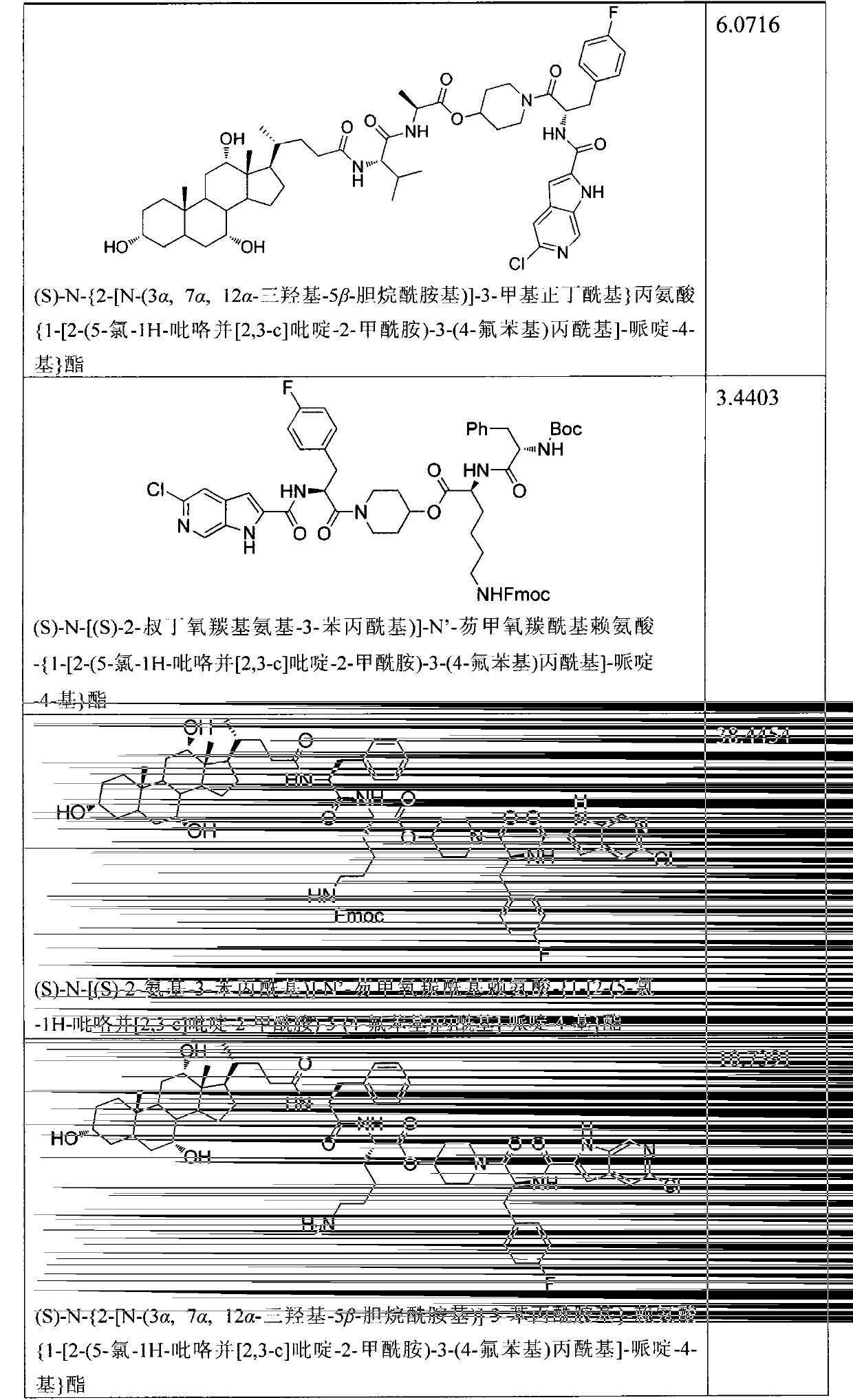
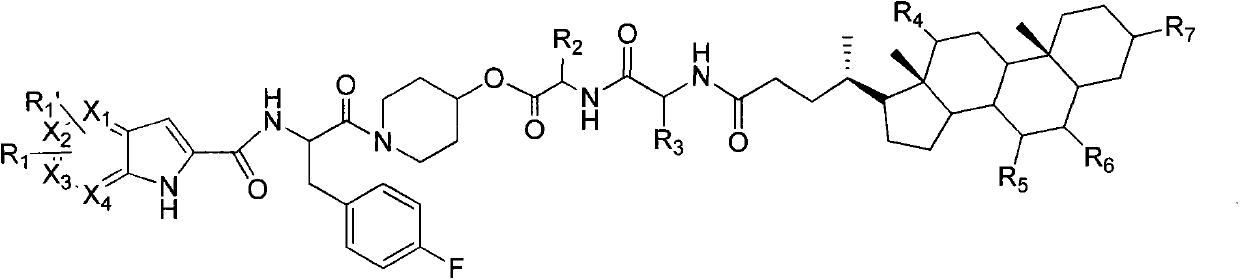
Glycogen phosphorylase inhibitor cholic acid derivative containing bio-cleavable dipeptide and preparation method and medical application thereof
A use and pharmaceutical technology, applied in the field of glycogen phosphorylase inhibitor bile acid derivatives, can solve the problems of high homology, muscle tissue myotoxicity, lack of liver glycogen phosphorylase selectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (S)-2-tert-butoxycarbonylamino-3-(4-fluorophenyl)-1-(4-hydroxypiperidin-1-yl)acetone
[0045] Dissolve BOC-4-fluoro-L-phenylalanine (15.6g, 55.1mmol) in anhydrous dichloromethane (160mL), add HATU (25g, 66.1mmol) and DIPEA (8.54g, 66.1 mmol), stirred at room temperature for 10 minutes, then added 4-hydroxypiperidine (6.7 g, 66.1 mmol), stirred at room temperature overnight. The reaction solution was washed with saturated brine, anhydrous Na 2 SO 4 It was dried, filtered and concentrated, and the residue was separated by reverse phase HPLC to give a white solid (50mg, 29.8%). Flash column chromatography (petroleum ether / ethyl acetate l / l, V / V) gave a white solid (19.7 g, 98%).
[0046] ESI-MS m / z: 367.2[M+H] + .
[0047] 1 H NMR (CDCl 3 , 400MHz): 7.14-7.18(m, 2H), 6.95-7.00(m, 2H), 5.47(dd, J=8.8, 14.8Hz, 1H), 4.83(dd, J=6.0, 13.6Hz, 1H), 3.81-4.01(m, 2H), 3.46-3.62(m, 1H), 3.15-3.33(m, 1H), 2.89-3.00(m, 2H), 1.73-1.83(m, 2H), 1.42-1.52(m , 2H), 1.40(s, 9H).
...
Embodiment 2
[0075] (S)-2-[(S)-2-tert-butoxycarbonylamino-3-phenylpropanylamino]-6-(benzyloxyamido)hexanoic acid methyl ester
[0076] In a similar manner to the preparation of (S)-2-(2-tert-butoxycarbonylamino-3-methyl-n-butyramide)propionic acid methyl ester, lysine methyl ester hydrochloride (2.49 g, 7.6 mmlo) Reaction with N-tert-butoxycarbonyl-L-phenylalanine (2 g, 7.6 mmol) gave a white solid (3 g, 73%).
[0077] ESI-MS m / z: 542.3[M+H] + .
[0078] 1 H-NMR (CDCl 3 , 400MHz): 7.38(d, J=4.4Hz, 4H), 7.29-7.35(m, 5H), 7.20-7.27(m, 3H), 6.49(d, J=7.2Hz, 1H), 5.11(dd, J=12.4, 16.4Hz, 2H), 4.54-4.59(m, 1H), 4.35-4.40(m, 1H), 3.72(s, 3H), 3.16(dd, J=6.0, 12.0Hz, 2H), 3.07 (t, J=6.0Hz, 2H), 1.77-1.88(m, 1H), 1.64-1.71(m, 1H), 1.49-1.56(m, 2H), 1.40(s, 9H), 1.27-1.36(m , 2H).
[0079] (S)-2-[(S)-2-tert-butoxycarbonylamino-3-phenylpropanylamino]-6-(benzyloxyamido)hexanoic acid
[0080] According to the similar method of preparing (S)-2-(2-tert-butoxycarbonylamino-3-methyl n-butyramide) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com