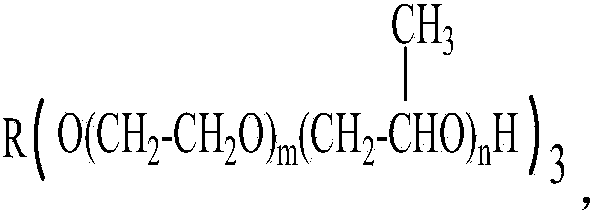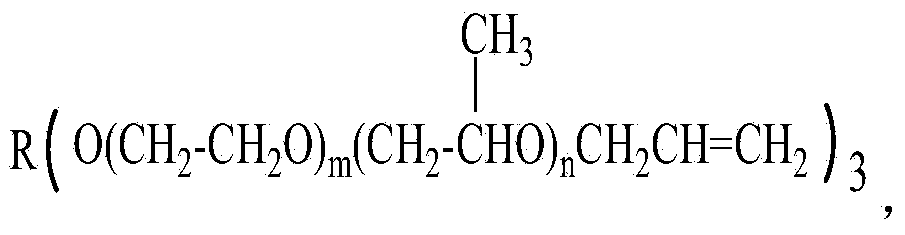Method for synthesizing trimethyl allyl polyoxypropylene ether
A technology of trimethylallyl polyoxypropylene ether and alcohol polyoxypropylene ether, which is applied in the field of preparation of alkyl-terminated polyethers, can solve the problem of highly toxic by-product allyl alcohol, increased side reactions, and capping rate Reduce and other problems, achieve the effect of high end-capping rate, fast reaction speed and high end-capping rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 1000 grams of glycerol polyoxypropylene ether (n=10, molecular weight about 1994) and 97.5 grams of solid sodium methoxide into the 2L reaction kettle. 0.09~-0.1MPa) methanol removal (removal of methanol is accompanied by the whole process of alkoxide reaction, and alkoxide is generated while removing methanol), and alkoxide reaction is carried out for 3 hours; the temperature is lowered to 70~80℃, and 177.0 grams of methyl Allyl chloride was maintained at a pressure of 0.3 MPa, continued to react for 4 hours, and the end-capping reaction was completed; excess methallyl chloride was recovered by distillation under reduced pressure, and the crude product was adsorbed and filtered by a polyether refining adsorbent to obtain a refined product.
Embodiment 2
[0042] Add 1000 grams of glycerol polyoxyethylene ether (n=45, molecular weight about 8084) and 30.1 grams of sodium methoxide into the 2L reaction kettle. After stirring, the temperature is controlled at 110~120℃, under vacuum conditions (pressure-0.09 ~-0.1MPa) methanol removal, alkoxide reaction for 3 hours; cooling to 80 ~ 90 ℃, feeding 53.7 grams of methallyl chloride, maintaining the pressure of 0.3Mpa, continuing the reaction for 5 hours, the end capping reaction is over; decompression Excess methallyl chloride is recovered by distillation, and the crude product is adsorbed and filtered by a polyether refining adsorbent to obtain a refined product.
Embodiment 3
[0044] Add 1000 grams of glycerol polyoxypropylene ether (n=85, molecular weight about 15044), 64.7 grams of liquid sodium methoxide (30% sodium methoxide methanol solution) into the 2L reaction kettle, stir evenly, and control the temperature at 120~130℃ , under vacuum conditions (pressure -0.09~-0.1MPa), methanol was removed, and the alkoxide reaction was carried out for 4 hours; the temperature was lowered to 90~100℃, 34.3 grams of methallyl chloride was introduced, the pressure was maintained at 0.3Mpa, and the reaction was continued for 5 hours , the end-capping reaction ends; the excess methallyl chloride is recovered by distillation under reduced pressure, and the crude product is adsorbed and filtered by a polyether refining adsorbent to obtain a refined product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com