Electrolyte for lithium secondary battery and lithium secondary battery
A lithium secondary battery, electrolyte technology, applied in secondary batteries, secondary battery repair/maintenance, electrolyte battery manufacturing, etc., can solve the problems of inability to reduce lithium metal electrochemical side reactions and limited solubility, etc. Achieve the effect of preventing direct contact, reducing irreversible side reactions, and improving cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
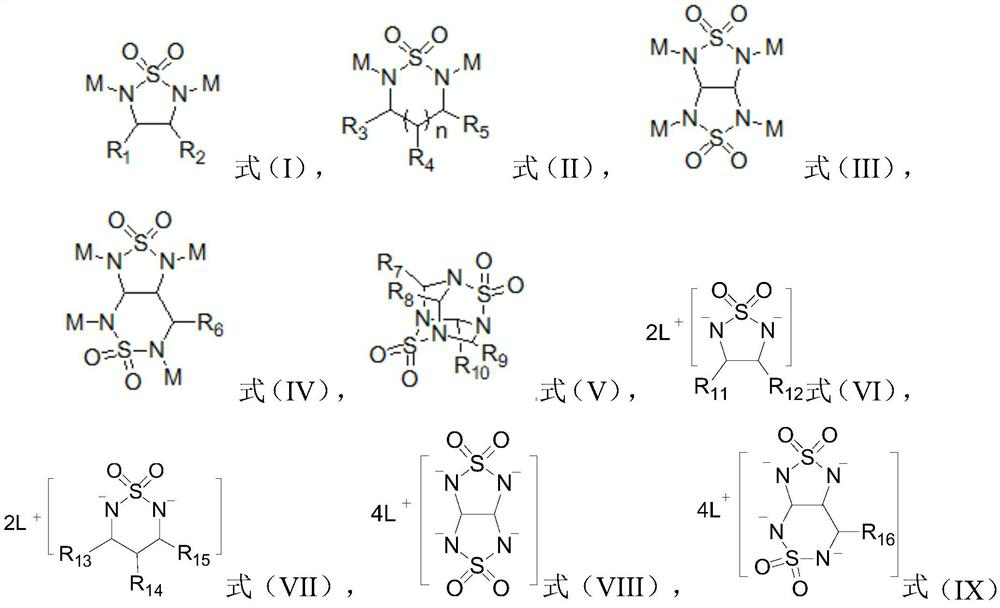
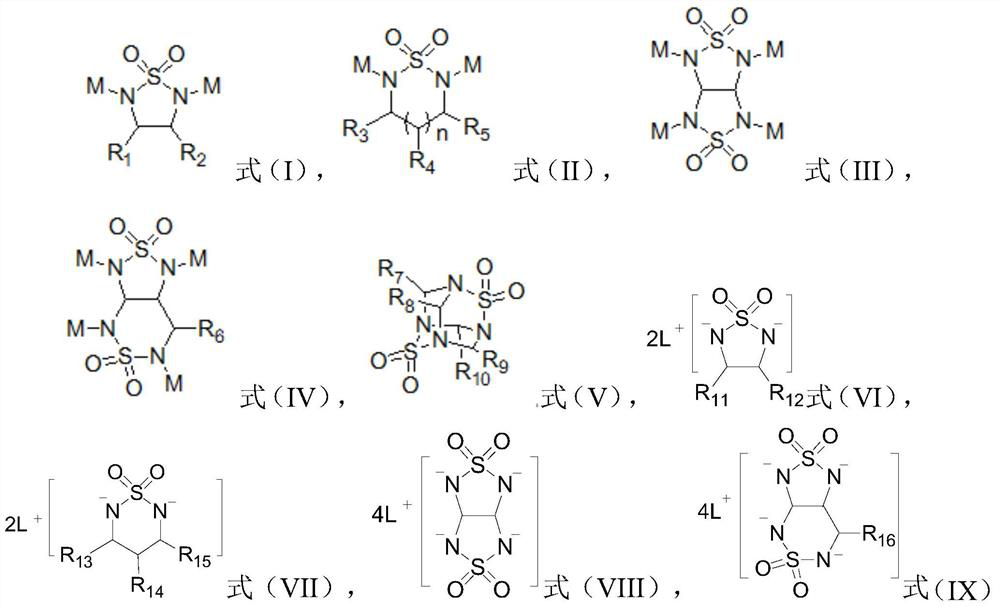
[0020] According to a preferred embodiment of the present invention, the alkali metal ions are selected from cesium or silver, which is more conducive to the shielding effect of alkali metal ions on the tip of lithium dendrites, effectively guiding Li + Deposits away from the Li dendrite tip, guiding the Li + Even deposition.
[0021] Preferably, the alkyl group is selected from at least one of straight-chain alkyl, branched-chain alkyl and heteroatom-containing alkyl, wherein the heteroatom is selected from at least one of F, N, O and S; Further preferably, the alkyl group is selected from methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, methoxy, ethoxy, fluoromethoxy, thiomethyl, At least one of methylamine and fluoromethyl. The use of a preferred alkyl group can effectively prevent molecular accumulation of the heterocyclic compound in the solution and improve the solubility of the additive.
[0022] Preferably, the halogen is selected from fluorine, chlorine, brom...
Embodiment 1
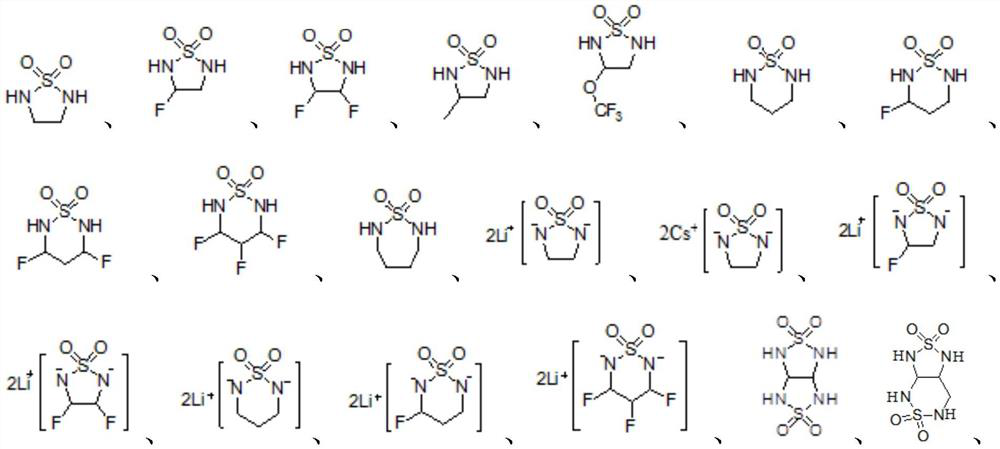
[0054] Electrolyte preparation : Add an organic solvent containing ethylene glycol dimethyl ether (DME) and 1,3-dioxolane (DOL) at a volume ratio of 50:50, and 1 mol / L based on the total amount of non-aqueous electrolyte amount of LiTFSI as a lithium salt, and then, based on the total amount of the non-aqueous electrolyte, 0.5% by weight of 1,2,5-thiadiazoline-1,1-dioxide as an electrolyte additive was added Electrolyte S1 is obtained.
[0055] Preparation of lithium secondary battery :
[0056] (1) By adding 98% by weight of Li(Ni) as a positive active material to N-methyl-2-pyrrolidone as a solvent 0.33 co 0.33 mn 0.33 )O 2 , 1% by weight of carbon black as a conductive agent and 1% by weight of polyvinylidene fluoride as a binder to prepare positive electrode slurry. The positive electrode slurry was then coated on an aluminum current collector with a thickness of 50 μm to form a positive electrode film, and dried and then rolled to prepare a positive electrode.
...
Embodiment 2
[0060] Electrolyte preparation : Addition of ethylene glycol dimethyl ether (DME) and 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (TTE) in a volume ratio of 50:50 An organic solvent, and LiFSI as a lithium salt in an amount of 2 mol / L based on the total amount of the non-aqueous electrolyte, and then, based on the total amount of the non-aqueous electrolyte, 0.5% by weight of 1,2, 5-Thiadiazoline-1,1-dioxo-2,5-dilithium salt Electrolyte S2 is obtained.
[0061] The preparation of the lithium secondary battery was the same as that in Example 1 to obtain the lithium secondary battery SP2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com