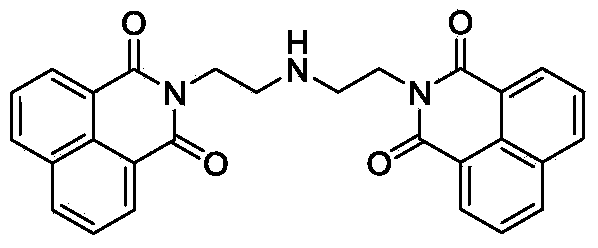
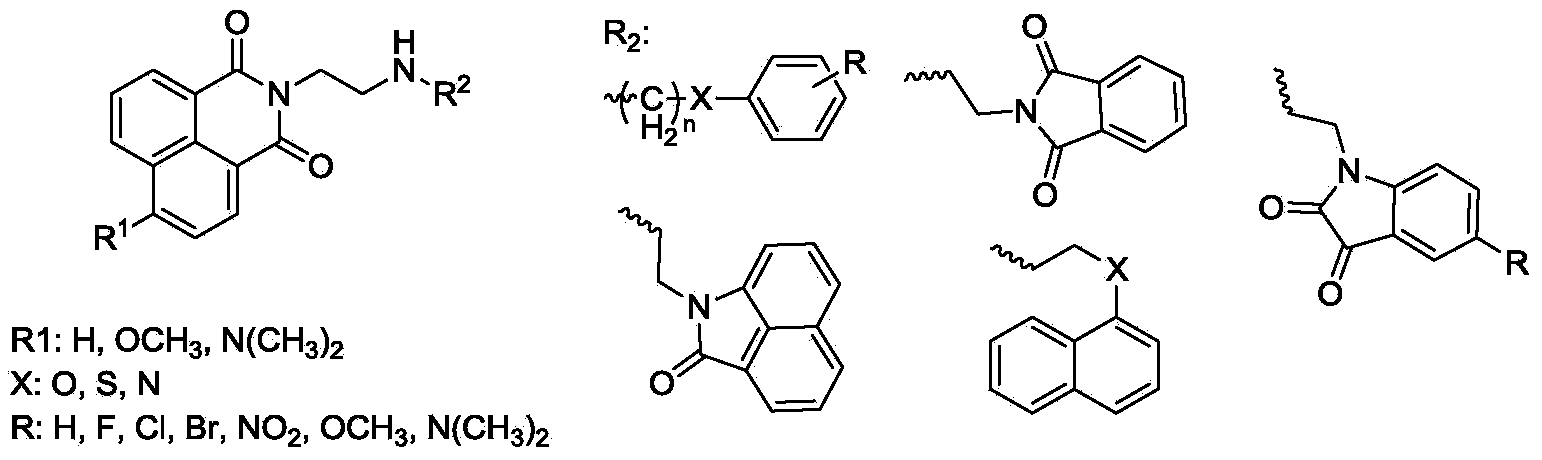
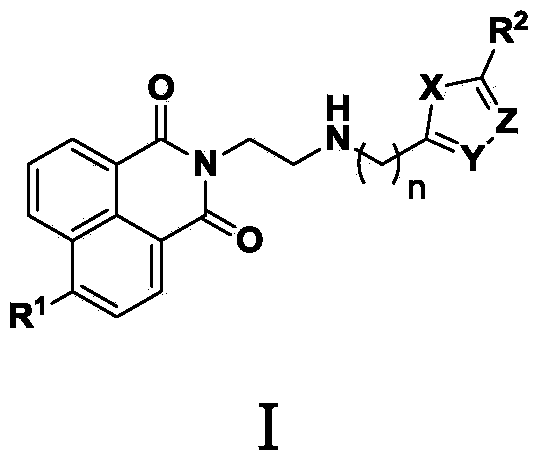
Naphthalimide derivative and application thereof as enzyme inhibitor and pesticide
A technology of naphthalimide and derivatives is applied in the directions of insecticides, biocides, animal repellents, etc., and achieves the effects of good application prospects, simple synthesis and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 2-(2-(((5-Methyl-1,3,4-thiadiazol-2-yl)methyl)amino)ethyl)-1H-benzo[de]isoquinoline-1,3 Synthesis of (2H)-diketone (1)
[0025]
[0026] Potassium carbonate (383 mg, 2.77 mmol) was added to 2-(2-aminoethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (500 mg, 2.08 mmol) and 2-chloro A solution of methyl-5-methyl-1,3,4-thiadiazole (206 mg, 1.39 mmol) in 20 mL of acetonitrile was heated under reflux and stirred until the reaction of the starting materials was completed by TLC monitoring, about 12 hours. The reaction solution was suction filtered to remove insoluble matter. The filtrate was evaporated to dryness under reduced pressure, and the residue was separated by silica gel column chromatography (CH 2 Cl 2 / CH 3 OH (30:1)) to give a white solid (250 mg, 0.71 mmol, 51%).
[0027] 1 H NMR (400MHz, CDCl 3 ):δ=8.59(d,J=7.2Hz,2H),8.23(d,J=8.0Hz,2H),7.76(dd,J=8.0,7.2Hz,2H),4.37(t,J=6.0Hz ,2H),4.22(s,2H),3.10(t,J=6.0Hz,2H),2.63(s,3H),1.96ppm(br,1H); 13 C NMR (100MHz, ...
Embodiment 2
[0029] 2-(2-(((5-Methyl-1,3,4-oxadiazol-2-yl)methyl)amino)ethyl)-1H-benzo[de]isoquinoline-1,3 Synthesis of (2H)-diketone (2)
[0030]
[0031] Except replacing 2-chloromethyl-5-methyl-1,3,4-thiadiazole in Example 1 with 2-chloromethyl-5-methyl-1,3,4-oxadiazole, Other conditions and steps were the same as in Example 1 to obtain compound 2 as a white solid with a yield of 50%.
[0032] 1 H NMR (400MHz, CDCl 3 ):δ=8.59(d,J=7.2Hz,2H),8.23(d,J=8.0Hz,2H),7.76(dd,J=8.0,7.2Hz,2H),4.37(t,J=6.0Hz ,2H),4.05(s,2H),3.10(t,J=6.0Hz,2H),2.47(s,3H),1.96ppm(br,1H); 13 C NMR (100MHz, CDCl 3 ): δ=172.0, 165.4, 164.1, 134.1, 131.6, 131.3, 128.2, 127.0, 122.5, 47.1, 43.4, 39.5, 11.0ppm; HRMS-ESI(m / z): Calculated value C 18 H 17 N 4 O 3 [M+H] + , 337.1301; experimental value, 337.1300.
Embodiment 3
[0034] 6-(Dimethylamino)-2-(2-(((5-methyl-1,3,4-thiadiazol-2-yl)methyl)amino)ethyl)-1H-benzo[ Synthesis of de]isoquinoline-1,3(2H)-dione (3)
[0035]
[0036] Divide by 2-(2-aminoethyl)-6-(dimethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione to replace Except for 2-aminoethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione, other conditions and steps were the same as in Example 1 to obtain compound 3, yellow oil, yield 50 %.
[0037] 1 H NMR (400MHz, CDCl 3 ):δ=8.54(dd,J=7.2,0.8Hz,1H),8.47-8.40(m,2H),7.64(dd,J=8.4,7.2Hz,1H),7.10(d,J=8.4Hz, 1H), 4.33(t, J=6.4Hz, 2H), 4.21(s, 2H), 3.10(s, 6H), 3.07(t, J=6.4Hz, 2H), 2.62(s, J=3H), 2.16ppm(br,1H); 13 C NMR (100MHz, CDCl 3):δ=172.1,165.6,164.8,164.2,157.0,132.7,131.3,131.1,130.3,125.2,124.8,122.9,114.7,113.2,47.8,47.3,44.7,39.4,15.5ppm;HRMS-ESI(m / z ): Calculated value, C 20 H 22 N 5 O 2 S[M+H] + , 396.1494; experimental value, 396.1494.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com