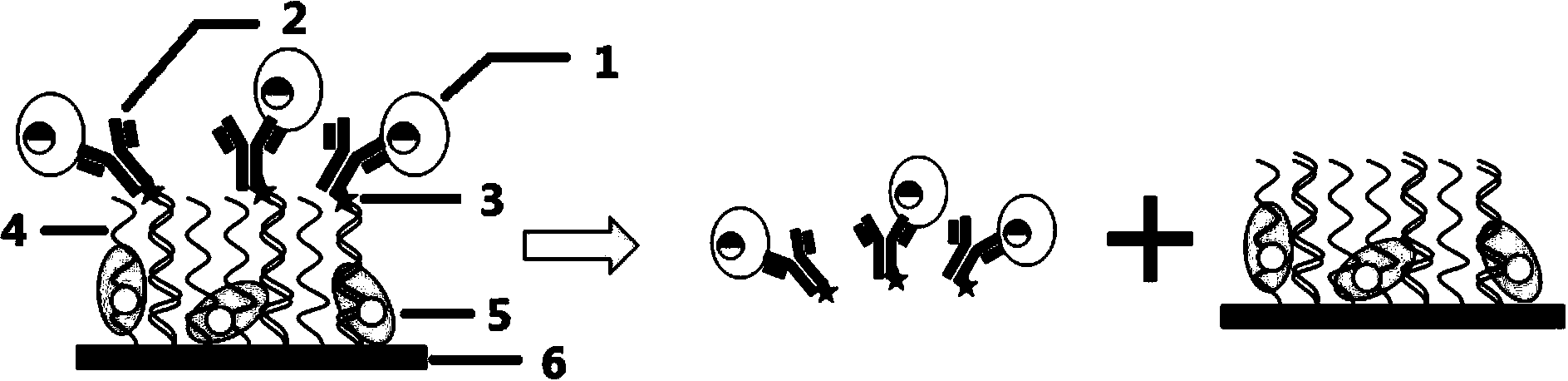
Method for separating high-purity circulating tumor cells from blood
A tumor cell, high-purity technology, applied in the field of biological and medical detection, can solve the problems of non-specific adsorption, time-consuming and labor-intensive, low purity of circulating tumor cells, etc., and achieves the effect of simple operation, high cutting efficiency and large exposure area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Release of Circulating Tumor Cells by Photosensitive Group and Ultraviolet Light Irradiation
[0042] M: AAAAAAAAAAGTCGAGGATTCTGAACCTGT;
[0043] M'-PC-NH 2 :
[0044] 5'-NH 2 - AAAAA-PCspacer-AAAAAACAGGTTCAGAATCCTCGAC-3';
[0045] M'-5PC-NH 2 :
[0046] 5, -NH 2 -PCspacer-AAAAAAAAAAACAGGTTCAGAATCCTCGAC-3'.
[0047] Wherein M is the polynucleotide loaded on the inner surface of the microchannel (see SEQ ID NO.1), M'-PC-NH- 2 (see SEQ ID NO.4) and M'-5PC-NH 2 (See SEQ ID NO.5) is a labeled single-stranded polynucleotide linked to the antibody, which can specifically bind to M. The PC spacer is a photosensitive group, which can be broken under the irradiation of a certain wavelength of light, preferably with a wavelength of 365 nanometers. M'-PC-NH 2 With M'-5PC-NH 2 The difference is that the photosensitive group is directly connected to the 5-terminal antibody, and is separated from the 5-terminal antibody by several nucleotide molecules. These po...
Embodiment 2
[0053] Example 2 Release of Circulating Tumor Cells by Restriction Endonucleases
[0054] A-EcoR1 (see SEQ ID NO.2): 5'-AAAAAAAAAAAAGAGCTAAGTCCGTAGAATTCAAAAAAAAAAGAGCTAAGTCCGTAGAATTCAAAAAAAAAAAAAA-3';
[0055] A-EcoRI′-NH 2 (see SEQ ID NO.6):
[0056] 5'-NH 2 -AAAAAAAAAAGAATTCTACGGACTTAGCTCCAGGAT-3';
[0057] B-BanHI (see SEQ ID NO.3): 5'-AAAAAAAAAAAATTGAATCATGCCTAGGATCCAAAAAAAAAATTGAATCATGCCTAGGATCCAAAAAAAAAAAAA-3';
[0058] B-BamHI′-NH 2 : (see SEQ ID NO.7) 5'-NH 2 -AAAAAAAAAAAGGATCCTAGGCATGATTCAATGAGGC-3';
[0059] Wherein A-EcoRI and B-BamHI are polynucleotides loaded on the inner surface of the microchannel, and A-EcoRI'-NH 2 with B-BamHI′NH 2 It is a labeled single-stranded polynucleotide linked to an antibody. A-EcoRI and A-EcoRI′-NH 2 There is a sequence that can be cut by the restriction endonuclease EcoRI, B-BamHI and B-BamHI'-NH 2 There is a sequence that can be cleaved by the restriction endonuclease BamHI in .
[0060] The capture method of tumor cells...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com