Nucleating agent and preparation method thereof
A technology of nucleating agent and acylating agent, applied in the field of nucleating agent and its preparation, can solve the problems of poor compatibility of polylactic acid matrix, reduction of reagent addition, insufficient formation of crystal nuclei, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The present invention also provides a preparation method of the nucleating agent represented by the above formula (I), comprising the following steps:
[0035] Mixing 5-sulfoisophthalic acid with an acylating agent, heating for acylation reaction, and then adding benzyl alcohol for alcoholysis reaction to obtain a nucleating agent represented by formula (I);
[0036] Or mix 5-sulfoisophthalic acid with an acylating agent to carry out acylation reaction, then add benzyl alcohol to carry out alcoholysis reaction, then add M metal or a compound containing M to carry out a salt-forming reaction to obtain the formula ( I) the indicated nucleating agent;
[0037]
[0038] Wherein, M is H, an alkali metal atom or an alkaline earth metal atom, and n is the valence of M.
[0039] The present invention has no special limitation on the sources of all raw materials, which can be commercially available.
[0040] The 5-sulfoisophthalic acid is preferably prepared according to th...
Embodiment 1
[0065] 1.1 Add 10g of isophthalic acid into a 150ml three-necked round-bottomed flask equipped with a reflux condenser, then add 20ml of 20% oleum, and heat the oil bath to 185°C for 6 hours under stirring conditions. After the reaction, remove the oil The bath was cooled to room temperature, the reaction system was diluted with distilled water, and fully cooled to precipitate 5-sulfoisophthalic acid to obtain 5-sulfoisophthalic acid.
[0066] 1.2 Add the 5-sulfoisophthalic acid obtained in 1.1 to a 250ml round-bottomed flask, add 40ml of thionyl chloride, and heat under reflux at 80°C for 5 hours. After the reaction, remove the remaining thionyl chloride and turn the reaction flask Cool in an ice-water bath, slowly add 30ml of benzyl alcohol dropwise, and carry out alcoholysis reaction. During the alcoholysis reaction, control the reaction temperature at 0°C to 30°C. After reacting for 5 hours, separate and purify to obtain the formula (I) in which M is H The nucleating agent...
Embodiment 2
[0071] The dibenzyl isophthalate obtained in Example 1 is mixed with 40% potassium carbonate aqueous solution, and a salt-forming reaction is carried out until the pH value is neutral, separated by filtration, and M is shown in formula (I) of K. The nucleating agent is an aromatic sulfonic acid potassium salt compound, the structure of which is shown in formula (III).
[0072]
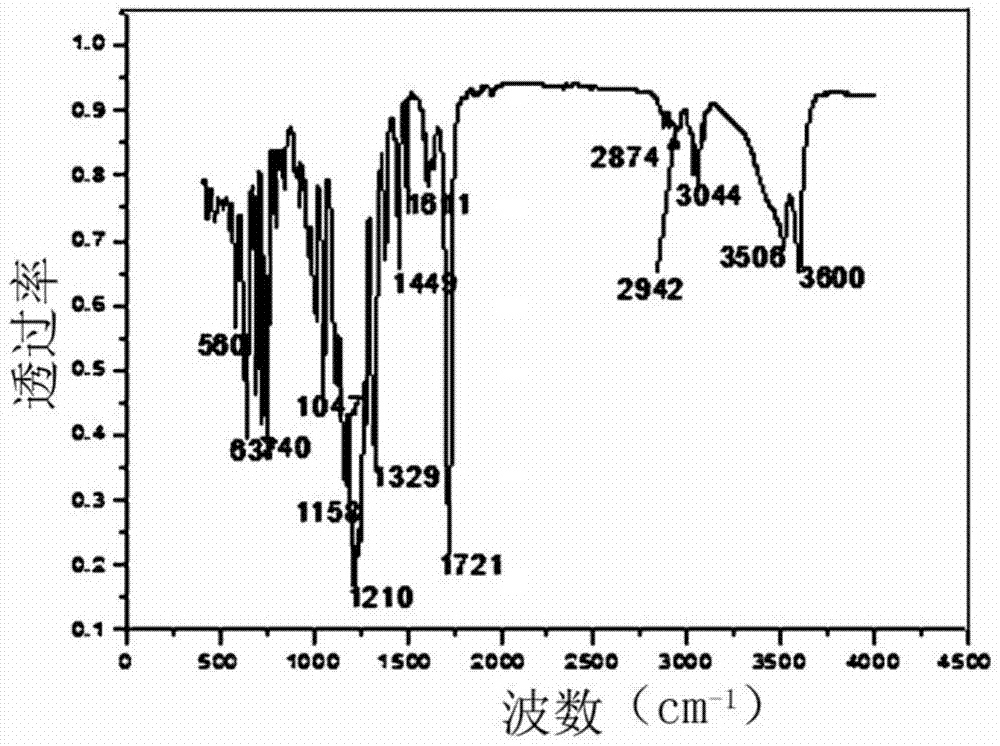
[0073] Utilize infrared spectrometer to analyze the aromatic sulfonic acid potassium salt compound obtained in embodiment 2, obtain its infrared spectrogram, as figure 1 shown. Depend on figure 1 It can be seen that 3044cm -1 The C-H stretching vibration peak of the benzene ring appears at 2942cm -1 and 2874cm -1 methylene-CH 2 The characteristic peak of -, 1721cm -1 The characteristic peak of ester C=O appears at 1611cm -1 、1508cm -1 and 1449cm -1 The characteristic vibration peak of the benzene ring skeleton appears at 1185cm -1 is the characteristic peak of the sulfo group.
[0074] Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half crystallization time | aaaaa | aaaaa |
| Half crystallization time | aaaaa | aaaaa |
| Half crystallization time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com