Rhamnose modified tumor whole-cell vaccine
A whole-cell vaccine, tumor cell technology, applied in the field of tumor whole-cell vaccine, can solve the problems of insufficient effect, complicated preparation process, no reports, etc., and achieves good application prospects, simple preparation method, and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
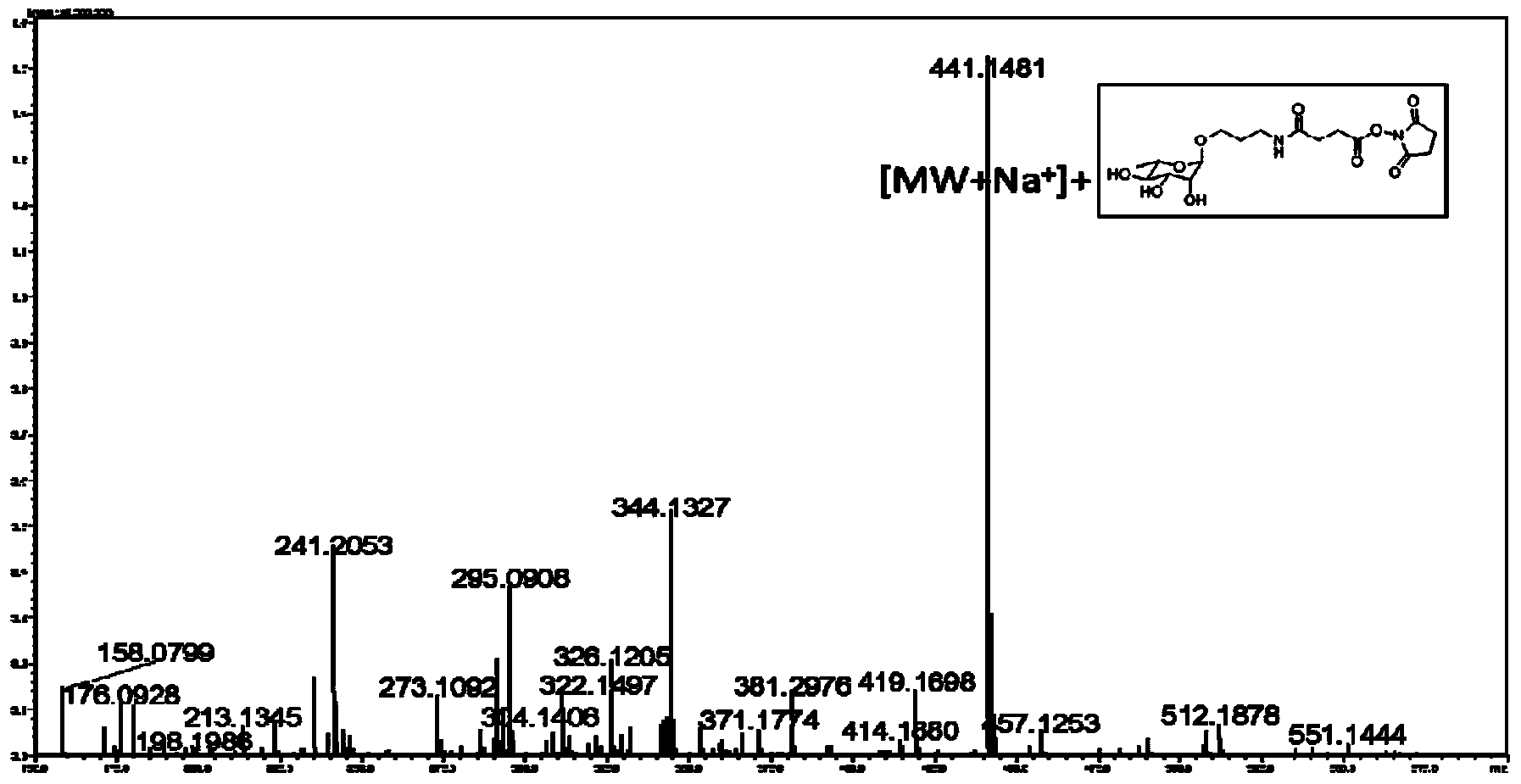
[0034] Embodiment 1: chemical synthesis activates Rha
[0035] 1. Dissolve 10g Rha in 100ml dichloromethane, add Ac 2 O3 1.1mL, Et 3 N6 1.2mL, DMAP 0.67g, stirred overnight at 0°C. Then 20ml of methanol was added to terminate the reaction, and the crude product of tetraacetate of Rha was obtained after washing and drying.
[0036] 2. Take out 24g of Rha tetraacetate and dissolve in 150ml of anhydrous methanol, add 8.12ml of thiophenol, SnCl 2 5.92ml, placed at 0°C and stirred for 3h. After washing, drying and concentrating, it is purified by column chromatography to obtain the glucosinolate compound of Rha.
[0037] 3. Take 1 g of the above glucosinolate compound, add 0.39 g of 3-azidopropanol, 0.88 g of NIS, 2-3 μl of TMSOTf, and react at -30°C for 1.5 h. Add 2-3ml NaHCO 3 Stop the reaction. After washing, drying, concentration and purification by column chromatography, an intermediate with an azide end is obtained.
[0038] 4. Dissolve 0.52g of the above-mentioned az...
Embodiment 2
[0043] Example 2: Preparation of Rha-modified human melanoma M21 whole cell vaccine
[0044] Different concentrations of activated Rha were prepared and mixed with M21 cells to prepare the Rha-modified human melanoma M21 tumor whole cell vaccine. Among them, the preferred cell concentration is 4×10 6 / ml, the final concentration of activated Rha was 1mg / ml.
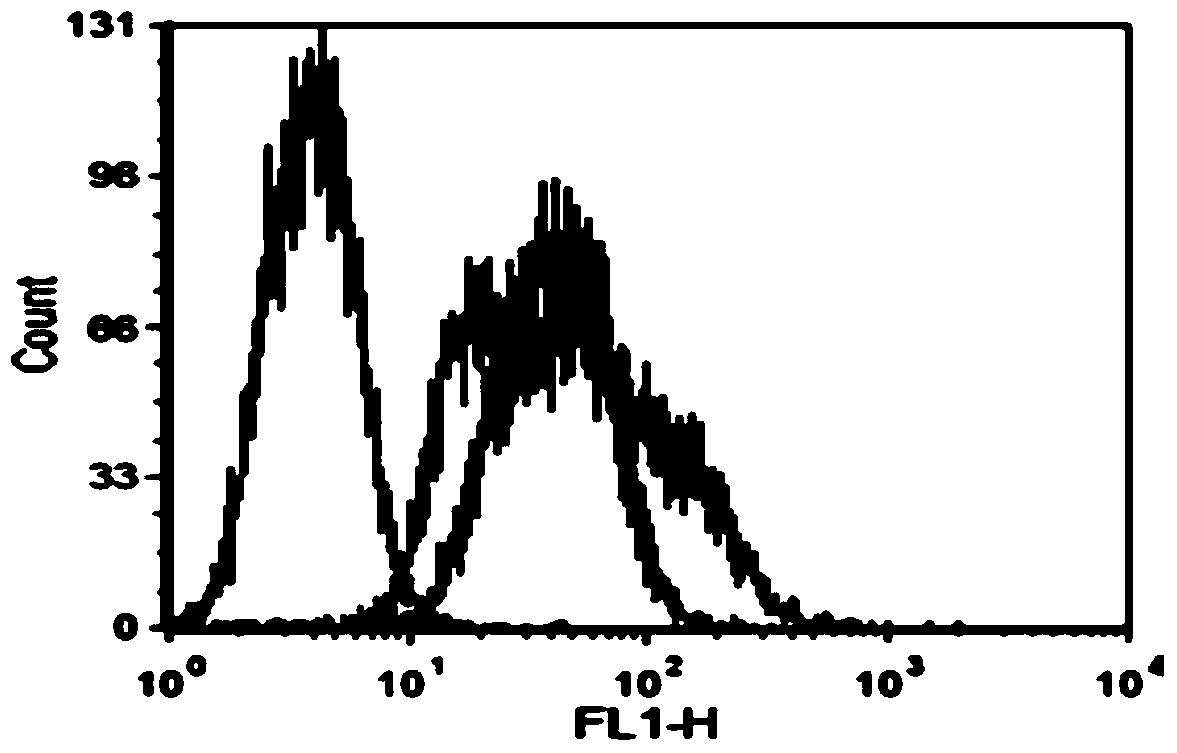
[0045] The reaction pH values were set to 7.4 and 8.5 respectively. After reacting at room temperature for 1 h, they were washed with PBS, and then FITC-labeled anti-rhamnose antibody was added. After washing, the efficiency of coupling Rha to M21 cells was detected by flow cytometry.
[0046] The results of flow cytometry showed that Rha was successfully coupled to the surface of M21 cells, ( figure 2 ) and when the reaction pH was 8.5, the proportion of M21 cells coupled with Rha reached over 92%, while when the pH was 7.4, the proportion of M21 cells coupled with Rha was only 75.92%.
[0047] The Rha-modified M21...
Embodiment 3
[0049] Example 3: Preparation of Rha-modified mouse melanoma B16 whole-cell vaccine
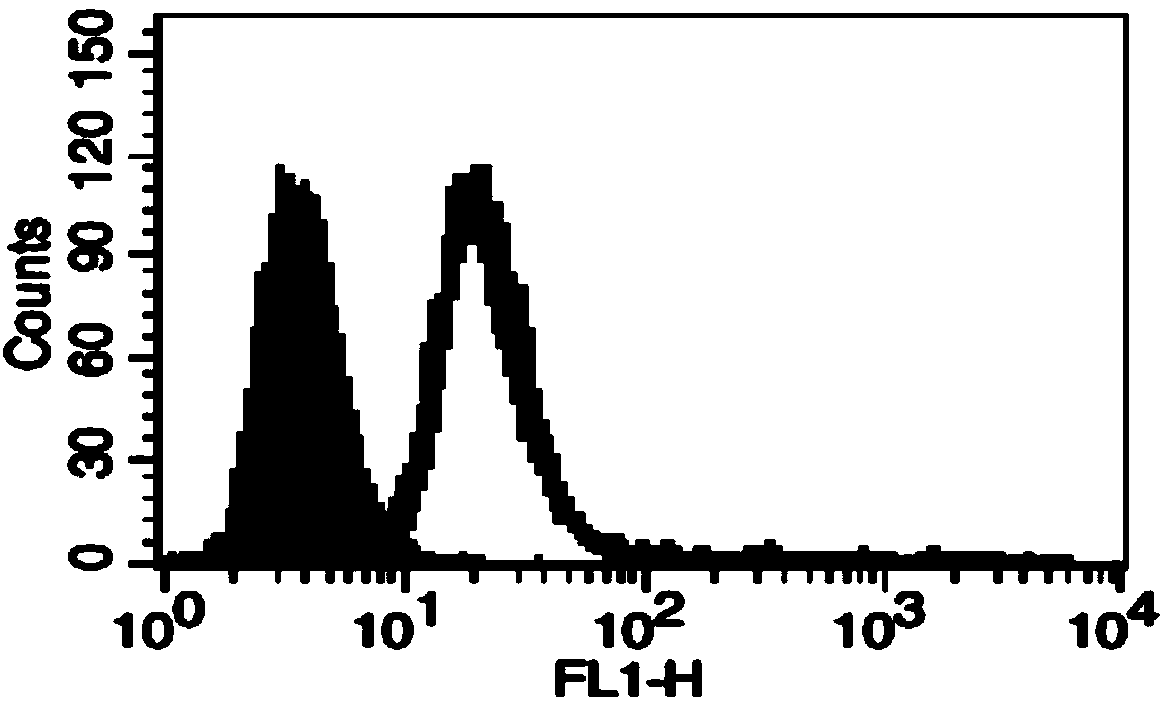
[0050] Different concentrations of activated Rha were prepared and mixed with B16 cells to prepare the Rha-modified mouse melanoma B16 tumor whole-cell vaccine. Among them, the preferred cell concentration is 4×10 6 / ml, the final concentration of activated Rha is 0.5mg / ml, 1mg / ml, 2mg / ml, 4mg / ml respectively, the reaction pH value is 8.5, after 1h reaction at room temperature, wash with PBS, then add FITC-labeled anti-rhamnose antibody , and then the efficiency of B16 cells coupled to Rha was detected by flow cytometry after washing.
[0051] The results of flow cytometry showed that Rha was successfully coupled to the surface of B16 cells, ( image 3 ) and when the activated Rha concentration was above 1 mg / ml, the proportion of B16 cells coupled with Rha reached over 98%.
[0052] The Rha-modified B16 tumor cells prepared above were irradiated with a total dose of 40Gy of X-rays to inac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com