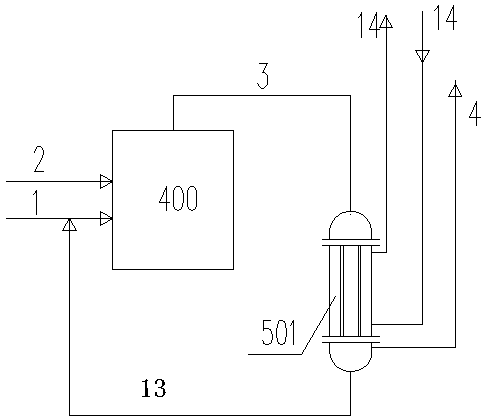Process for removing and recycling dimethyldichlorosilane from hydrochloric acid gas
A technology of dimethyldichlorosilane and hydrochloric acid gas, which is applied in the chemical field, can solve problems such as the impossibility of complete recovery, and achieve the effect of simple method, low operating cost, economical and practical
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
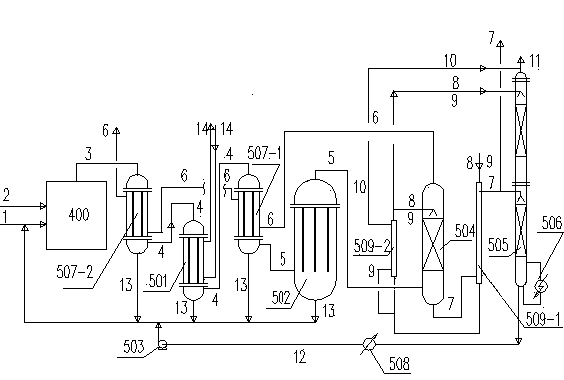
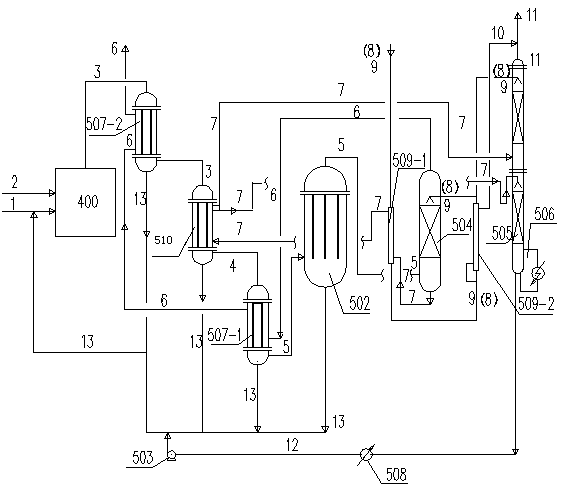
[0035] Use below figure 2 and image 3 Two examples are shown to further describe the invention.
[0036] refer to figure 2 , a kind of removing and reclaiming dimethyl dichlorosilane process from hydrochloric acid gas in embodiment 1 is to set the primary HCl cooler 507-1 in the downstream of the existing frozen brine graphite cooler 501, and the gas phase contains dimethyl The HCl gas 4 of dichlorosilane90% (wt) concentrated acid mist droplets and dimethyldichlorosilane small droplets suspended in the HCl gas phase, and gather into large droplets to slide down to the lower part of the glass wool filter 502 by gravity In the collection tank, the filtered dimethyldichlorosilane 2 O3 Cl and dry HCl are in countercurrent contact on the surface of the wire mesh corrugated packing, and liquid CH 3 Cl volatilizes continuously, and the temperature rapidly drops to the limit -70°C, and the liquid CH 3 The volatilization of Cl provides a large amount of cold temperature, so that...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com