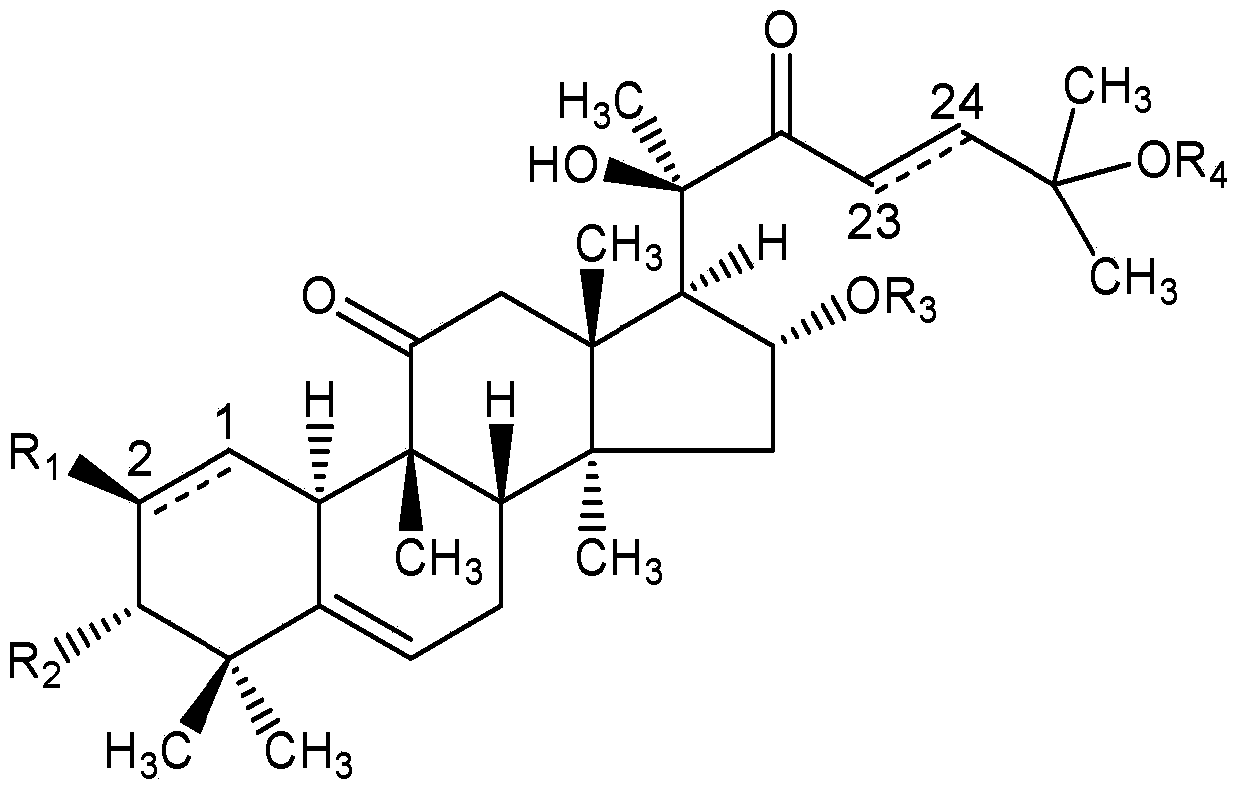
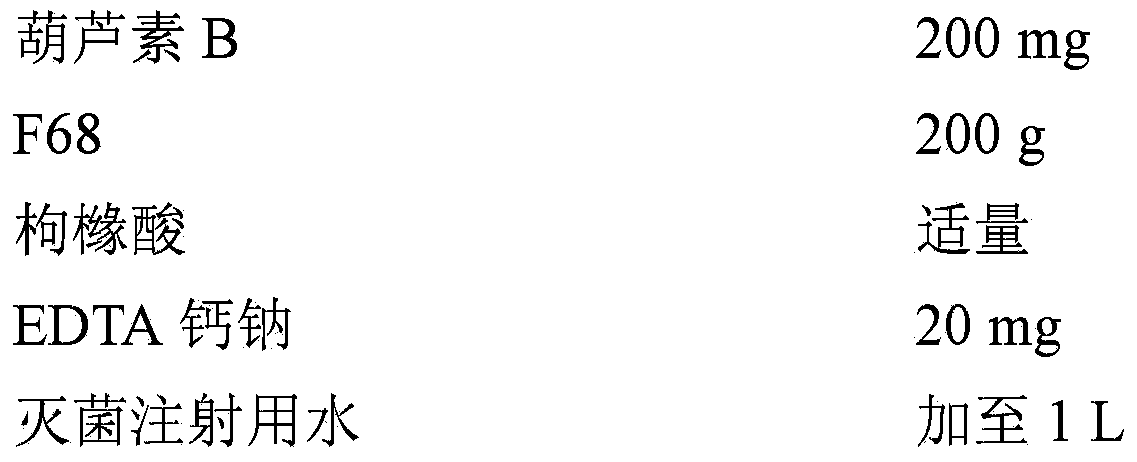Cucurbitacin medicinal composition and pharmaceutical application thereof
A composition, the technology of cucurbitacin, which is applied in the field of cucurbitacin pharmaceutical composition and its pharmaceutical use, to achieve the effect of reducing toxicity, excellent stability and enhancing curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of cucurbitacin B injection
[0032] prescription:
[0033]
[0034] Preparation method: Preheat sterilized water for injection at 70°C for later use; add the prescribed amount of F68 and cucurbitacin B into a beaker, stir at 70°C until completely dissolved, and continue stirring for 30 minutes; inject the same temperature under magnetic stirring Sterile water for injection (20 mg of calcium sodium EDTA is dissolved in it), stir until the system becomes a clear and transparent solution, then dilute to 1L with sterile water for injection, adjust the pH value to 6 with citric acid after mixing; add Appropriate amount of activated carbon, after stirring at room temperature for 30 minutes, filter to remove the activated carbon; pass through a 0.22 μm microporous membrane, dispense into 2 mL brown vials, 1 mL per bottle, fill with nitrogen, stopper, seal with an aluminum cap, sterilize at 121 ° C for 10 min, Instantly.
Embodiment 2
[0035] Embodiment 2: the preparation of cucurbitacin B injection
[0036] prescription:
[0037]
[0038] Preparation method: Weigh the prescribed amount of cucurbitacin B, polyethylene glycol 1000 lauryl hydroxystearate and stir evenly in a water bath at 70°C to form an oil phase; weigh the prescribed amount of F68, propylene glycol, ascorbic acid and part of water for injection Also heat to 70°C, after the substances in the two phases are completely dissolved, slowly add the water phase to the oil phase under stirring, continue stirring for 30 minutes, then dilute to 1L with water for injection, adjust the pH with sodium hydroxide after mixing value to 5; add an appropriate amount of activated carbon to it, stir at room temperature for 30 minutes, and filter to remove the activated carbon; pass through a 0.22 μm microporous membrane, dispense into 2 mL brown vials, each bottle is 1 mL, fill with nitrogen, stopper, and seal with an aluminum cap. Sterilize at 121°C for 10 ...
Embodiment 3
[0039] Embodiment 3: Preparation of cucurbitacin B freeze-dried preparation
[0040] prescription:
[0041]
[0042]
[0043] Preparation method: Weigh the prescribed amount of mannitol and EDTA calcium sodium, dissolve them completely at 70°C with 800mL sterile water for injection, preheat for later use, and obtain the aqueous phase; stir the prescribed amount of F68 and cucurbitacin B at 70°C until it is completely dissolved to obtain the oil phase; after the substances in the two phases are completely dissolved, slowly add the water phase to the oil phase under the condition of magnetic stirring, continue to stir for 30 minutes, dilute to 1L with sterile water for injection, mix well and use apple Adjust the pH value to 4.5 with acid; pass through a 0.22 μm microporous membrane, dispense into 3 mL brown vials, 1 mL each, freeze-dry, fill with nitrogen, stopper, and seal with an aluminum cap.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com