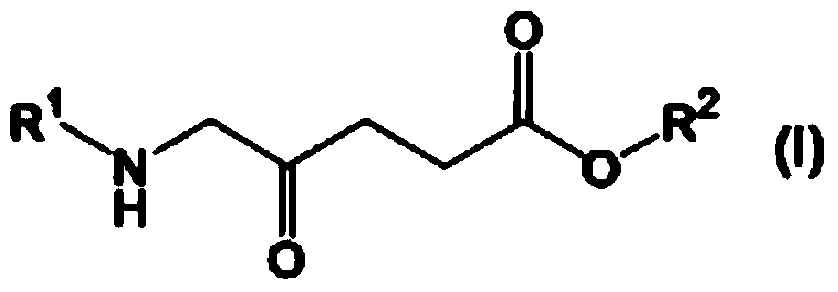
Prophylactic agent and/or therapeutic agent for sepsis
A technology for sepsis and preventive agents, applied in the field of preventive and/or therapeutic agents for sepsis, which can solve the problems of exacerbation of infectious diseases, unrealistic, and no progress in research, and achieve the goals of reducing side effects, improving drug efficacy, and excellent therapeutic effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
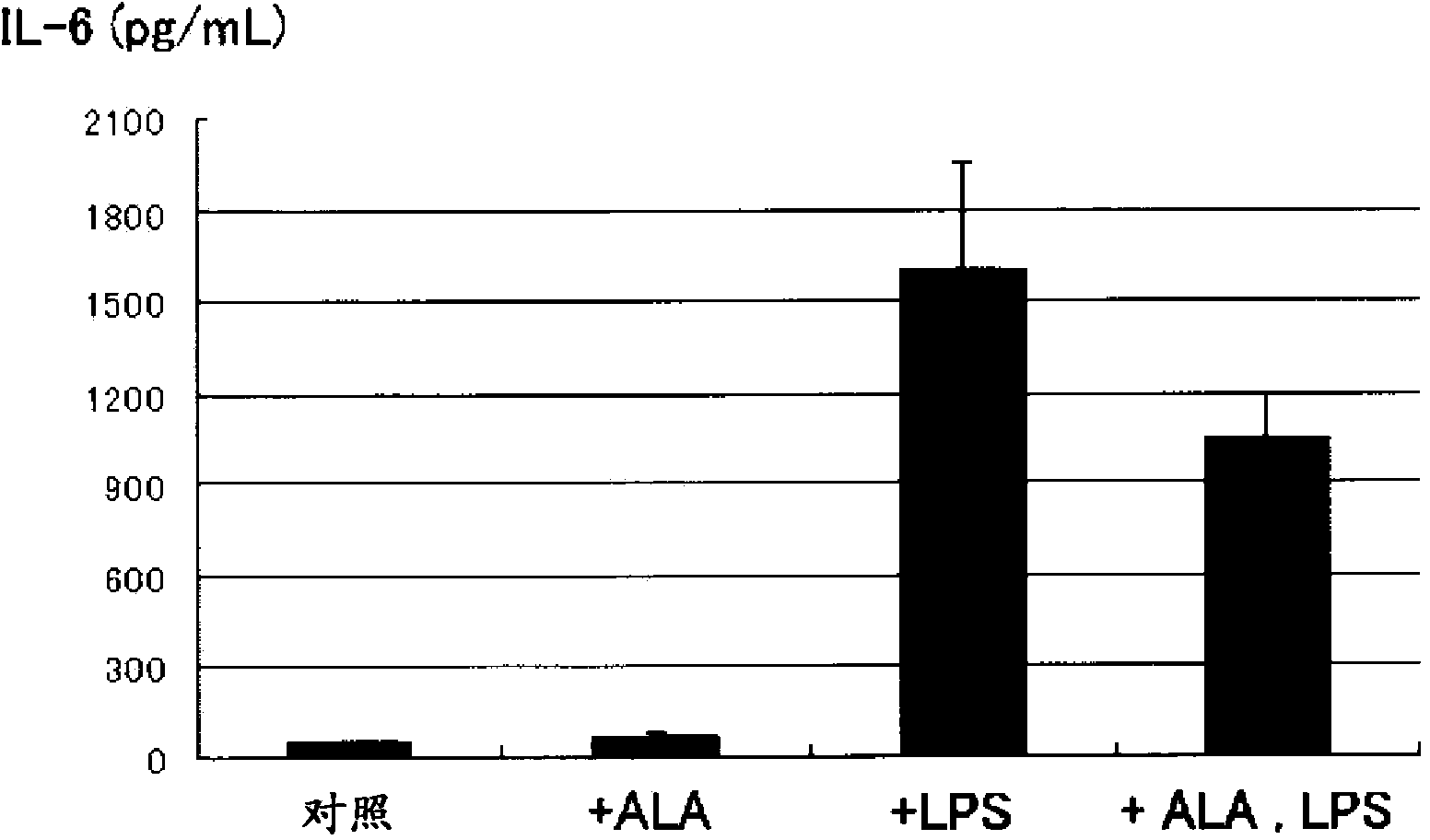
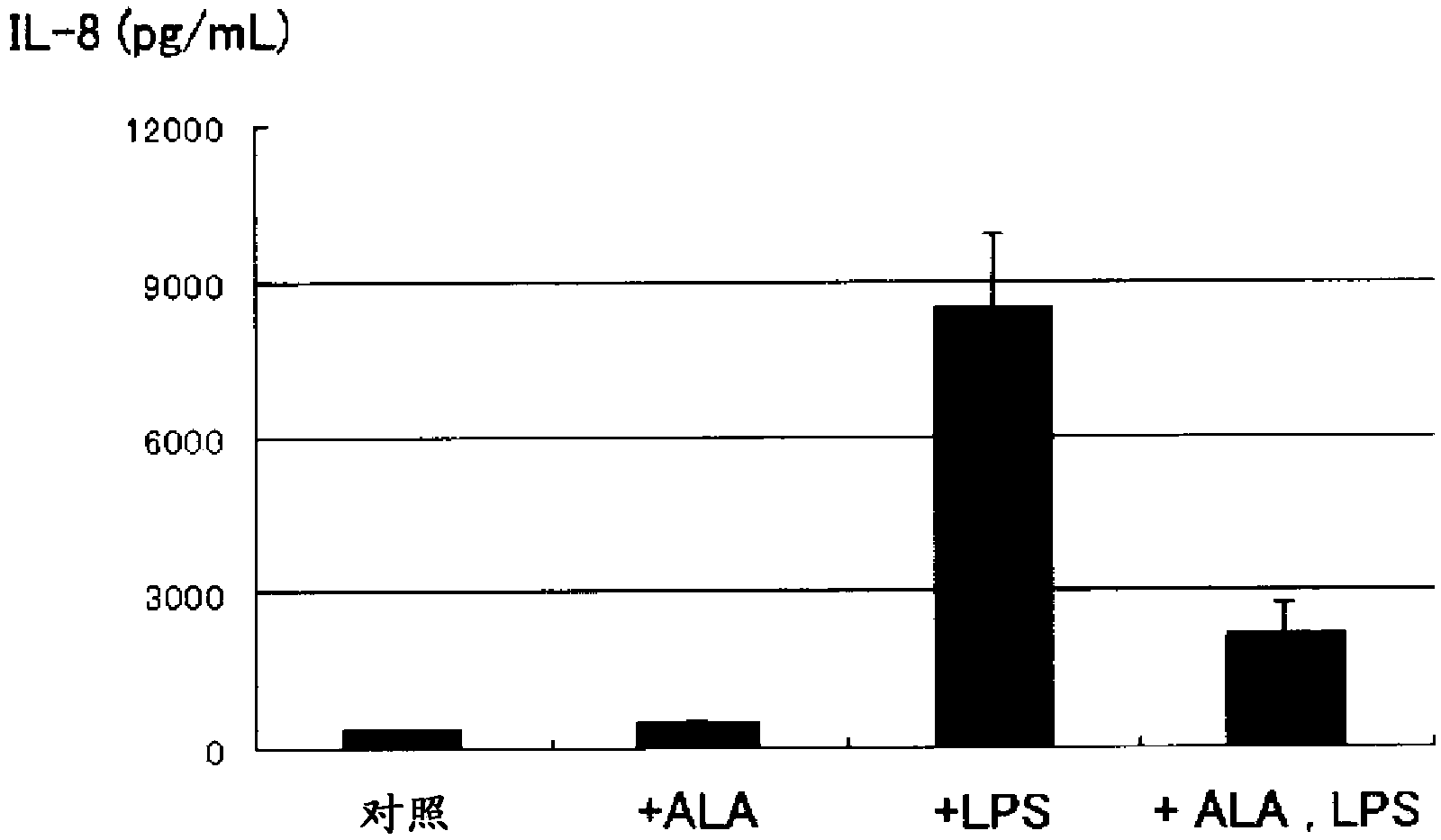
[0097] Using a sepsis model in which human pulmonary artery endothelial cells were mixed with lipopolysaccharide (LPS), which can promote the secretion of inflammatory cytokines, the ELISA method was used to measure IL-6 in the supernatant ( figure 1 ), IL-8 ( figure 2 ) for the following experiments. The organ with the highest frequency of damage in sepsis is the lung, so human pulmonary artery endothelial cells were used in this experiment.
[0098] Human pulmonary artery endothelial cells (5×10 5 cells / well, n=3 wells), and were divided into the following 4 groups according to whether LPS and 5-ALA were added.
[0099] (1) Control without stimulation (in the figure: control)
[0100] (2) Add 5-ALA (100μM) and mix for 3 hours (in the figure: +ALA)
[0101](3) Add LPS (1μg / mL) and mix for 3 hours (in the figure: +LPS)
[0102] (4) Add 5-ALA (100μM) and LPS (1μg / mL) and mix for 3 hours (in the figure: +ALA, LPS)
[0103] In the group to which 5-ALA was added, sodium fer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com