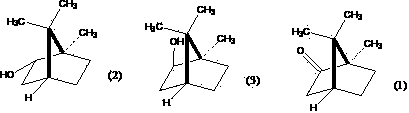
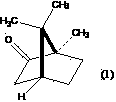
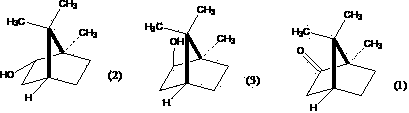
Method for preparing high-purity borneol from lauraceae extract or blumea balsamifera extract
A technology of extract and aina incense, applied in the fields of chemistry and medicine, can solve the problems of high price and short supply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1: Prepare high-purity d-borneol by natural camphor or its reduction product (1)-distillation-sublimation method
[0083] Take 17g of aluminum powder (AR), add 5g of anhydrous AlCl 3 (AR), quickly mix, then add 2.0gHgCl 2 (AR), mix well, add isopropanol (AR) 1000ml, under airtight, after about 20min, react rapidly, use condenser to condense if necessary. After 6 hours, react in a water bath for 4 hours, add 120 g of natural camphor, and distill off isopropanol in an air bath at 110°C. Add steam, distill, and dry at room temperature to obtain 100 g of natural camphor reduction products (D-borneol, D-isoborneol, a small amount of natural camphor mixture). Take 4g aluminum powder, add 3.5g anhydrous AlCl 3 Mix quickly, add 200ml of isopropanol, under airtight, reflux in a water bath for 12h, add 100g of the above-mentioned natural camphor reduction product, heat until the natural camphor reduction product dissolves, shake well to form a homogeneous solution. ...
Embodiment 2
[0084] Embodiment 2: Prepare high-purity D-borneol-column chromatography by natural camphor or its reduction products
[0085] Take 17g of aluminum powder, add 5g of anhydrous AlCl 3 , mix quickly, then add 2.0gHgCl 2 , mix well, add 1000ml of isopropanol, under airtight, after about 20min, react quickly, if necessary, use a condenser to condense. After 6 hours, react in a water bath for 4 hours, add 120 g of natural camphor, and distill off isopropanol in an air bath at 110°C. Steam distillation was introduced, condensed water was cooled, and the cooled product was subjected to airtight distillation to remove water to obtain 115 g of natural camphor reduction products (D-borneol, D-isoborneol, a small amount of natural camphor mixture). Take 4g aluminum powder, add 3.5g anhydrous AlCl 3 Mix quickly, add 200ml of isopropanol, under airtight, reflux reaction in a water bath for 12h, add 115g of the above-mentioned camphor reduction product, heat until the camphor reduction p...
Embodiment 3
[0086] Embodiment 3: Prepare high-purity d-borneol-extraction method by natural camphor or its reduced product
[0087] Take 17g of aluminum powder, add 5g of anhydrous AlCl 3 , mix quickly, then add 2.0gHgCl 2 , mix well, add 1000ml of isopropanol, under airtight, after about 20min, react quickly, if necessary, use a condenser to condense. After 6 hours, react in a water bath for 4 hours, add 120 g of natural camphor, and distill off isopropanol in an air bath at 110°C. Distilled with water steam, cooled with condensed water, and the cooled product was distilled to remove water in a closed manner to obtain 114g of natural camphor reduction products (D-borneol, D-isoborneol, a small amount of natural camphor mixture). Take 4g aluminum powder, add 3.5g anhydrous AlCl 3, Mix quickly, add 300ml of isopropanol, under airtight, reflux in a water bath for 12h, add 114g of the above-mentioned natural camphor reduction product, and heat until the natural camphor reduction product d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com