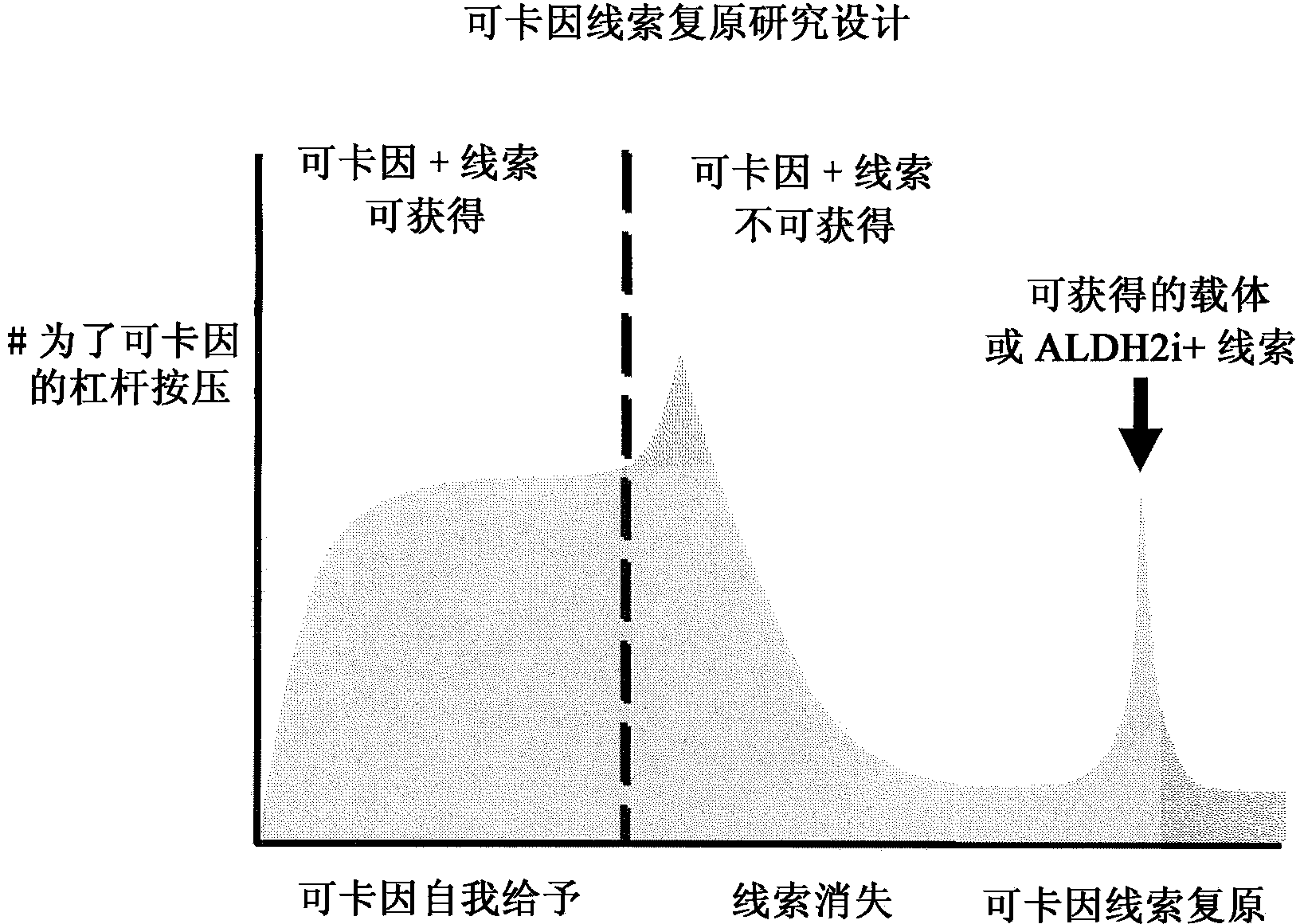
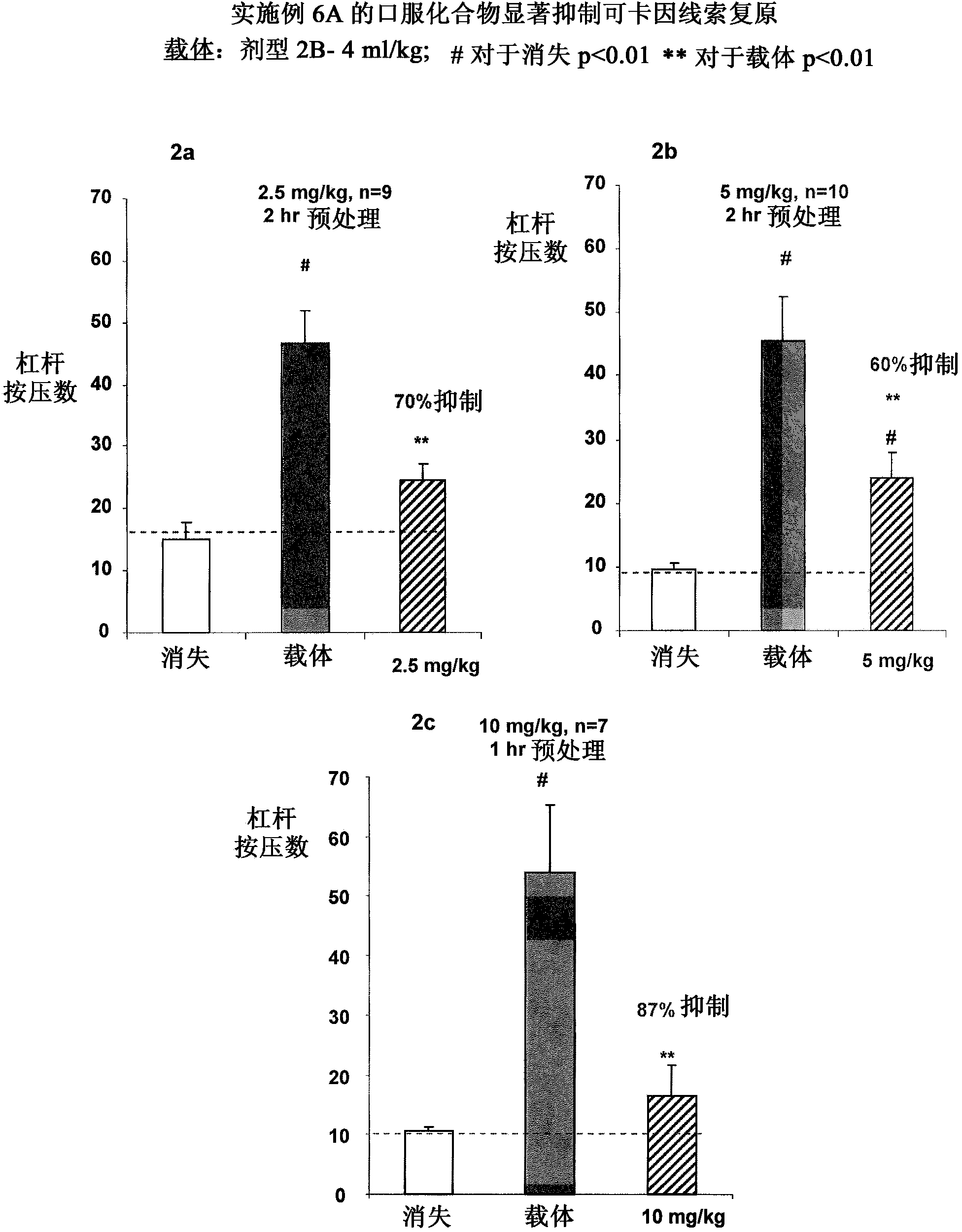
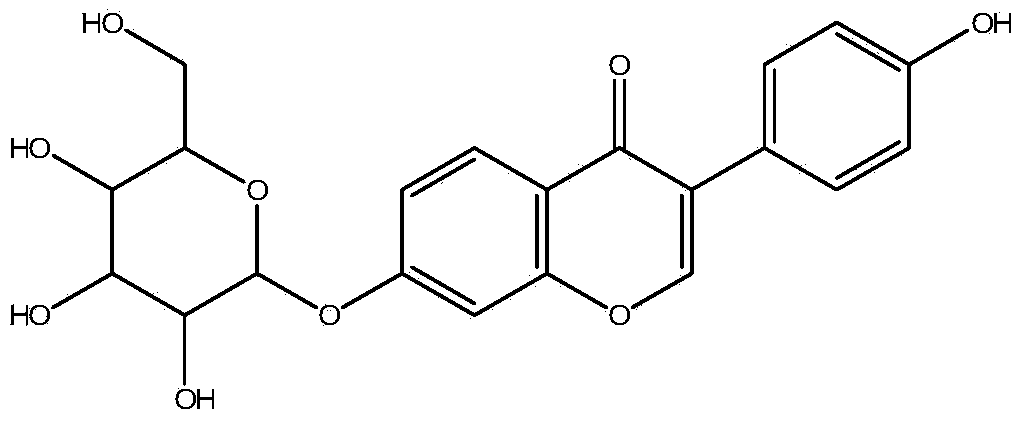
ALDH-2 inhibitors in the treatment of addiction
一种化合物、苯基的技术,应用在成瘾治疗中的ALDH-2抑制剂领域,能够解决没有证明等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0219] Preparation of compounds of formula (2)
[0220] a. prepared where R 3 is a compound of formula (2) that is hydrogen
[0221]
[0222] To 7-hydroxy-3-(4-nitrophenyl)-4H-chromen-4-one (7-hydroxy-3-(4-nitrophenyl)-4H-chromen-4-one) (commercially available, (10 g, 48.3 mmol)) in dichloromethane (100 mL), added pyridine (15.6 mL, 193.2 mmol), and cooled the mixture in an ice bath. To this solution was slowly added trifluoromethanesulfonic anhydride (16.3 mL, 96.6 mmol) at 0 °C, and then the solution was warmed to room temperature and stirred for 1 hour until the reaction was complete. The reaction mixture was partitioned between ethyl acetate and water. The organic phase was dried over magnesium sulfate and the solvent was removed under reduced pressure. The residue was heated in acetonitrile and the solid was filtered off to give 3-(4-nitrophenyl)-4-oxo-4H-chromen-7-yl trifluoromethanesulfonate (3-(4-nitrophenyl )-4-oxo-4H-chromen-7-yltrifluoromethanesulfonat...
Embodiment 2
[0224] Preparation of compounds of formula (3)
[0225] a. prepared where R 3 is a compound of formula (3) where hydrogen
[0226]
[0227] To a suspension of 3-(4-nitrophenyl)-4-oxo-4H-chromen-7-yl trifluoromethanesulfonate (5.0 g, 14.75 mmol) in tetrahydrofuran (20 mL) was added fresh A solution of sodium dithionite (5.13 g in 20 mL) was prepared. The mixture was stirred at room temperature for 2 hours, and then additional sodium dithionite (2.57 g) was added in two portions. The reaction mixture was stirred at room temperature for 24 hours, after which time the reaction was complete. The organic solvent was evaporated under reduced pressure, and then water (10 mL) was added to the suspension. The solid in the suspension was filtered off and dried under high vacuum. The product was heated with acetonitrile and stirred until room temperature was reached. The solid was filtered off to afford 3-(4-aminophenyl)-4-oxo-4H-chromen-7-yl trifluoromethanesulfonate (4.1 ...
Embodiment 3
[0229] Preparation of compounds of formula (4)
[0230] a. prepared where R 2 is methyl and R 3 is a compound of formula (4) where hydrogen
[0231]
[0232] 3-(4-Aminophenyl)-4-oxo-4H-chromen-7-yl trifluoromethanesulfonate (2.2 g, 7.12 mmol) in pyridine (10 mL) was dissolved within 5 min at 0 °C Methanesulfonyl chloride (1.1 mL, 14.24 mmol) was added to the suspension, and then allowed to warm to room temperature with stirring. After 2 hours, the reaction was complete and water was added in portions with vigorous stirring. The organic phase was then separated and concentrated, and the resulting solid was filtered and dried under high vacuum. The solid was heated in acetonitrile and stirred until room temperature was reached, then filtered off to give 3-(4-(methylsulfonylamino)phenyl)-4-oxo-4H-chromen-7-yltrifluoromethanesulfonic acid Esters (2.49g). 1 H NMR (DMSO, 400MHz) 9.87(s, 1H); 8.60(s, 1H); 8.31(d, J=8.0Hz, 1H); 8.09(s, 1H); 7.65(d, J=8.0Hz, 1H ); 7.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com