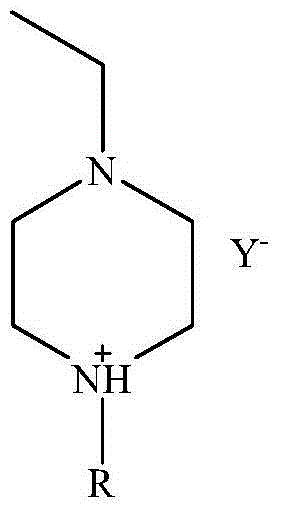
1-ethyl-4-alkylpiperazine ionic liquid and preparation method and application thereof
A technology of alkylpiperazine and ionic liquid, which is applied in the field of 1-ethyl-4-alkylpiperazine ionic liquid, can solve the problems of poor stability, few types, short preparation cycle, etc., and achieve easy functionalization and short cycle , the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
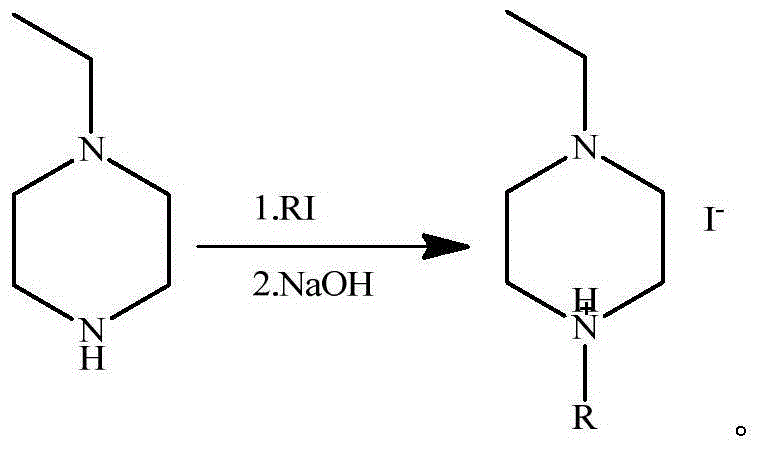
[0036] Embodiment 1: Preparation of iodide 1-ethyl-4-propylpiperazine ionic liquid
[0037] Dissolve 0.11 mol of iodopropane in 50 mL of dichloromethane, drop slowly into 0.1 mol of 1-ethylpiperazine, and react with stirring at 20°C for 20 hours. After the reaction was completed, 0.12 mol of sodium hydroxide was added to the reaction solution, and the reaction was continued at 20° C. for 24 hours. After the reaction, filter to remove solid impurities, evaporate dichloromethane, and wash the crude product twice with n-hexane, evaporate n-hexane, and dry under vacuum at 40°C to obtain 1-ethyl-4-propylpiperazine iodide ion Liquid, 98% purity, 90% yield.
[0038] The reaction formula is:
[0039]
Embodiment 2
[0040] Embodiment 2: Preparation of iodide 1-ethyl-4-butylpiperazine ionic liquid
[0041]Dissolve 0.1 mol of iodobutane in 50 mL of dichloromethane, drop slowly into 0.1 mol of 1-ethylpiperazine, and react with stirring at 5°C for 24 hours. After the reaction was completed, 0.14 mol of sodium hydroxide was added to the reaction liquid, and the reaction was continued at 25° C. for 24 hours. After the reaction, filter to remove solid impurities, evaporate dichloromethane, wash the crude product twice with n-hexane, evaporate n-hexane, and dry under vacuum at 40°C to obtain 1-ethyl-4-butylpiperazine iodide Liquid, 99% purity, 86% yield.
[0042] The reaction formula is:
[0043]
Embodiment 3
[0044] Embodiment 3: Preparation of iodide 1-ethyl-4-hexylpiperazine ionic liquid
[0045] Dissolve 0.09 mol of iodohexane in 50 mL of dichloromethane, drop slowly into 0.1 mol of 1-ethylpiperazine, and react with stirring at 40°C for 24 hours. After the reaction was completed, 0.12 mol of sodium hydroxide was added to the reaction liquid, and the reaction was continued at 40° C. for 24 hours. After the reaction, remove solid impurities by filtration, evaporate dichloromethane, wash the crude product twice with n-hexane, evaporate n-hexane, and dry under vacuum at 40°C to obtain 1-ethyl-4-hexylpiperazine iodide ionic liquid , 98% purity, 88% yield.
[0046] The reaction formula is:
[0047]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com