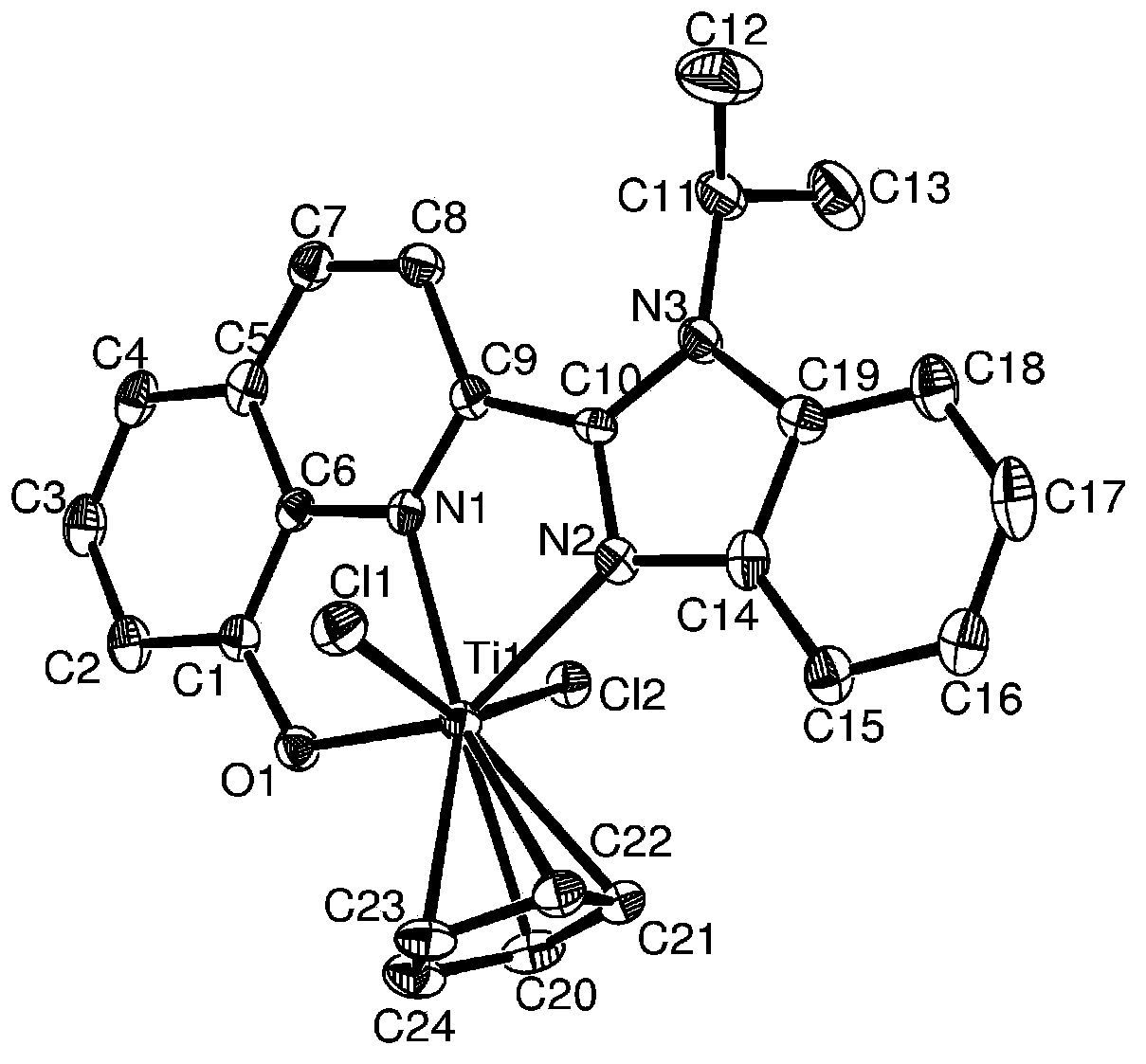
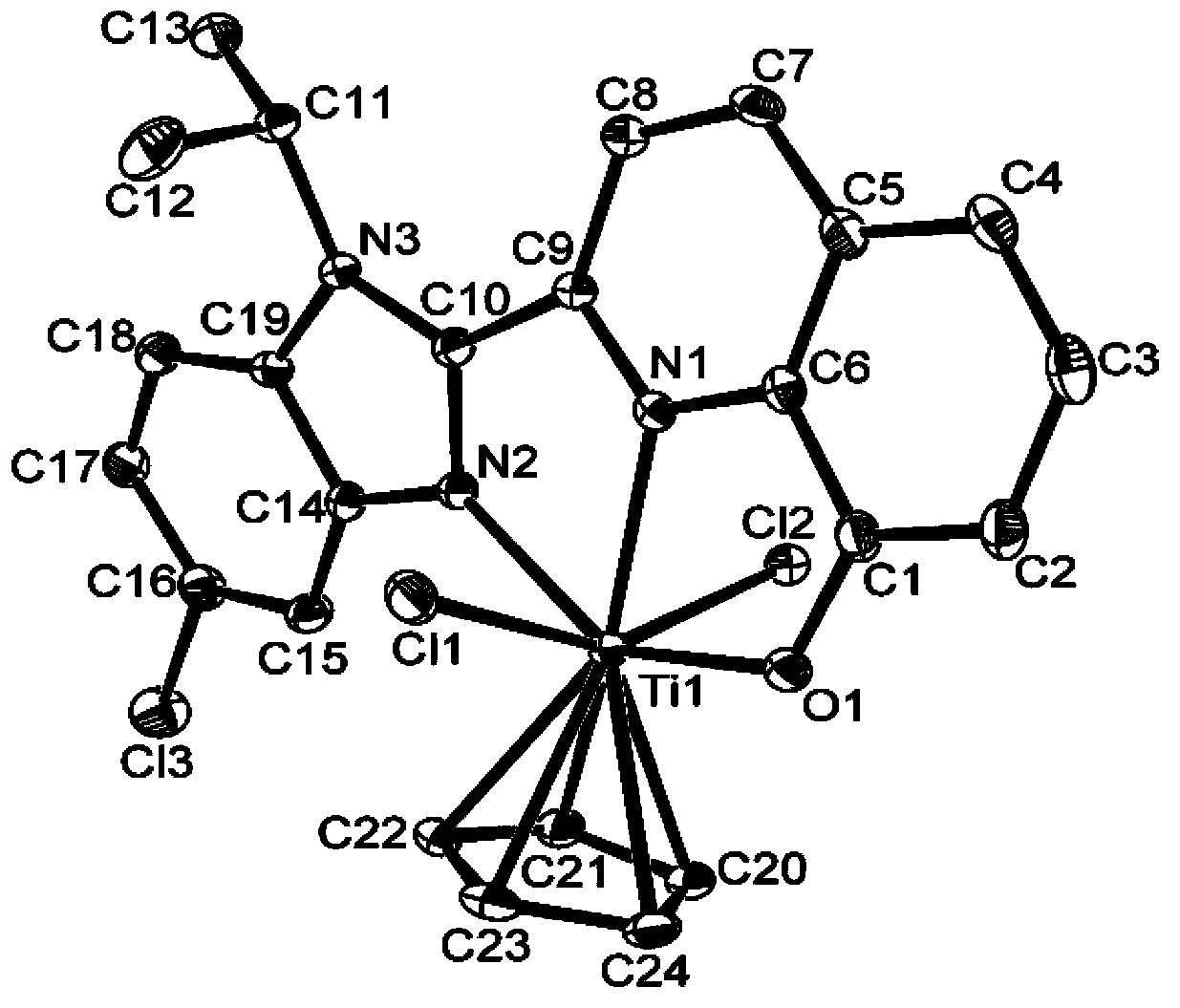
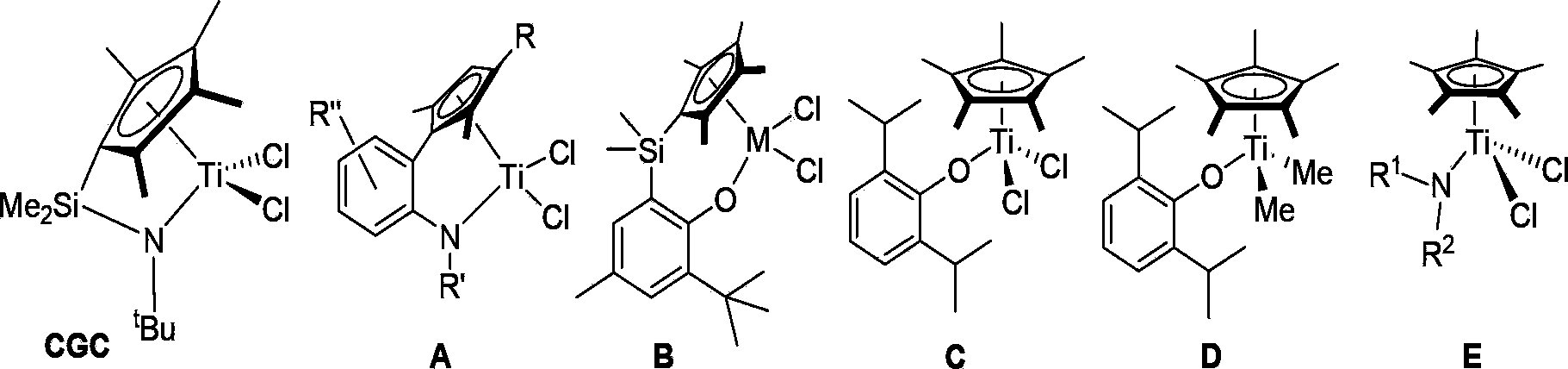
Quinoline-benzimidazole IVB group mono-Cp complex, preparation method and application thereof and method for polymerization reaction of olefin
A technology of benzimidazoles and IVB family, applied to quinoline benzimidazole family IVB monocene complexes and their preparation and application, and the fields of olefin polymerization, can solve the problem of high price and inability to control the form of polymer products well , a large number of problems, etc., to achieve the effect of high catalytic reaction activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] This example is used to illustrate the quinoline benzimidazole IVB group monocene complex and its preparation method provided by the present invention.
[0076] At -78°C, 4 ml of n-BuLi in hexane (concentration: 0.5 mol / L) was added to a solution containing 2-N-methylbenzimidazole-8-hydroxyquinoline (0.551 g, 2 mmol). Chloromethane (30mL) solution, then raised to 10°C at a rate of 5°C / min and stirred for 4 hours to generate a yellow precipitate of 2-N-methylbenzimidazole-8-hydroxyquinoline lithium salt. Filter and drain the precipitate, add 30 ml of dichloromethane and mix well, then add an equivalent amount of Cp'TiCl at -78°C 3(0.438g, 2.00mmol, Cp' is cyclopentadienyl), and then raised to 10°C at a rate of 5°C / min and stirred for 24 hours. After the solvent was drained, use CH 2 Cl 2 (5×20mL) extract the product and concentrate, and then recrystallize with n-heptane to obtain (2-N-methylbenzimidazole-8-hydroxyquinoline) cyclopentadienyl monocene titanocene complex ...
Embodiment 2
[0083] This example is used to illustrate the quinoline benzimidazole IVB group monocene complex and its preparation method provided by the present invention.
[0084] At 0°C, 4 ml of n-BuLi in hexane (concentration: 0.5 mol / L) was added to a dichloride solution containing 2-N-ethylbenzimidazole-8-hydroxyquinoline (0.579 g, 2 mmol). methane (30mL) solution, then raised to 25°C at a rate of 2°C / min and stirred for 4 hours to generate a yellow precipitate of 2-N-ethylbenzimidazole-8-hydroxyquinoline lithium salt. Filter and drain the precipitate, add 30 ml of dichloromethane and mix well, then add an equivalent amount of Cp'TiCl at 0°C 3 (0.438g, 2.00mmol, Cp' is cyclopentadienyl), and then raised to 25°C at a rate of 2°C / min and stirred for 24 hours. After the solvent was drained, use CH 2 Cl 2 (5×20mL) extract the product and concentrate, and then recrystallize with n-heptane to obtain (2-N-ethylbenzimidazole-8-hydroxyquinoline) cyclopentadienyl titanocene complex (wherein, ...
Embodiment 3
[0091] This example is used to illustrate the quinoline benzimidazole IVB group monocene complex and its preparation method provided by the present invention.
[0092] At -50°C, 4 ml of n-BuLi in hexane (concentration: 0.5 mol / L) was added to a solution containing 2-N-isopropylbenzimidazole-8-hydroxyquinoline (0.606 g, 2 mmol). Dichloromethane (30mL) solution, then raised to 15°C at a rate of 3°C / min and stirred for 4 hours to generate a yellow precipitate of 2-N-isopropylbenzimidazole-8-hydroxyquinoline lithium salt. Filter and drain the precipitate, add 30 ml of dichloromethane and mix well, then add an equivalent amount of Cp'TiCl at -50°C 3 (0.438g, 2.00mmol, Cp' is cyclopentadienyl), and then raised to 15°C at a rate of 3°C / min and stirred for 24 hours. After the solvent was drained, use CH 2 Cl 2 (5×20mL) extract the product and concentrate, and then recrystallize with n-heptane to obtain (2-N-isopropylbenzimidazole-8-hydroxyquinoline) cyclopentadienyl titanocene compl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com