Crystalline form of thymidine phosphorylase inhibitor and preparation method thereof
A technology of trifluorothymidine and crystal form, applied in the directions of organic chemistry, organic chemistry, pharmaceutical formulations, etc., to achieve the effects of short cycle, good stability and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0044] Preparation Example 1: Preparation of 5-chloro-6-(1-(2-iminopyrrolidinyl)methyl)uracil hydrochloride
[0045] A. Preparation of 5-chloro-6-chloromethyluracil:
[0046]
[0047] Add 100ml of acetic acid to 20.0g of 6-chloromethyluracil, add 22ml of sulfuryl chloride dropwise within 20 minutes under stirring condition, after the dropwise addition is completed, stir and react at 30-35°C, TLC monitors that the reaction of raw materials is complete, and slowly pour the reaction solution into 200ml Suction filter in ice water, wash with 10-15ml of methanol, and air-dry at 50°C to obtain 16.0g of off-white solid with a yield of 66.0%.
[0048] ESI-MS (m / z): [M+H] 195.0; 1 HNMR (600MHz, DMSO-d 6 , δppm): 4.47 (S, 2H), 11.58 (S, 1H), 11.72 (S, 1H).
[0049] b. Preparation of 5-chloro-6-(1-(2-iminopyrrolidinyl)methyl)uracil hydrochloride
[0050]
[0051] In the reaction bottle, add 150ml N, N-dimethylformamide (DMF), 19.5g 2-aminopyrrolidine hydrochloride, 18.0g sodium...
Embodiment 1
[0053] Example 1: Preparation of 5-chloro-6-(1-(2-iminopyrrolidinyl)methyl)uracil hydrochloride crystal form I
[0054] Add 1.0 g of the solid product obtained by the method of Preparation Example 1 into a mixed solvent composed of 3.0 ml of water and 15 ml of isopropanol, heat and stir until dissolved, then cool down to 15°C within 1.5 to 2 hours while stirring, continue stirring for 2 hours, and filter. After washing and drying, 0.94 g of the crystal form I described in the title was obtained in the form of 0.94 g of white crystals, with a yield of 94% and a purity of 100.0% by HPLC;
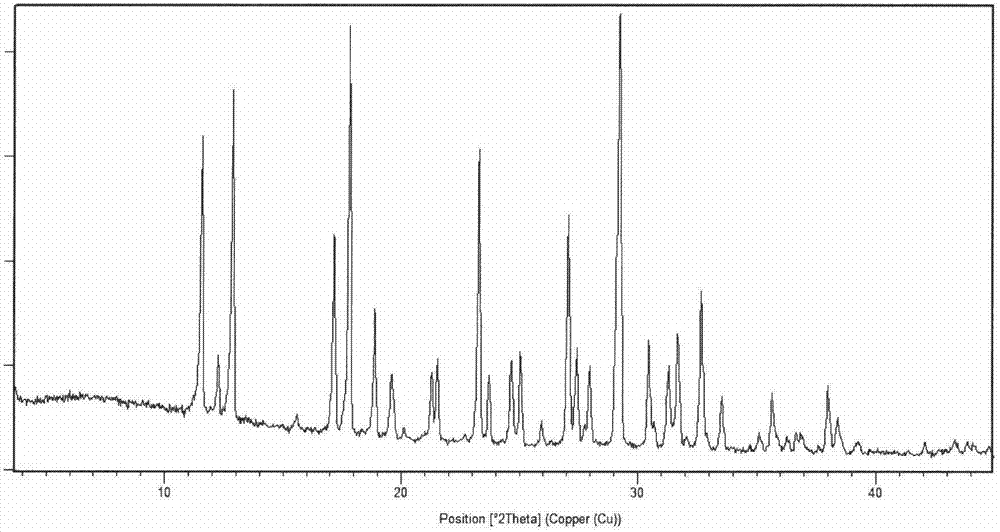
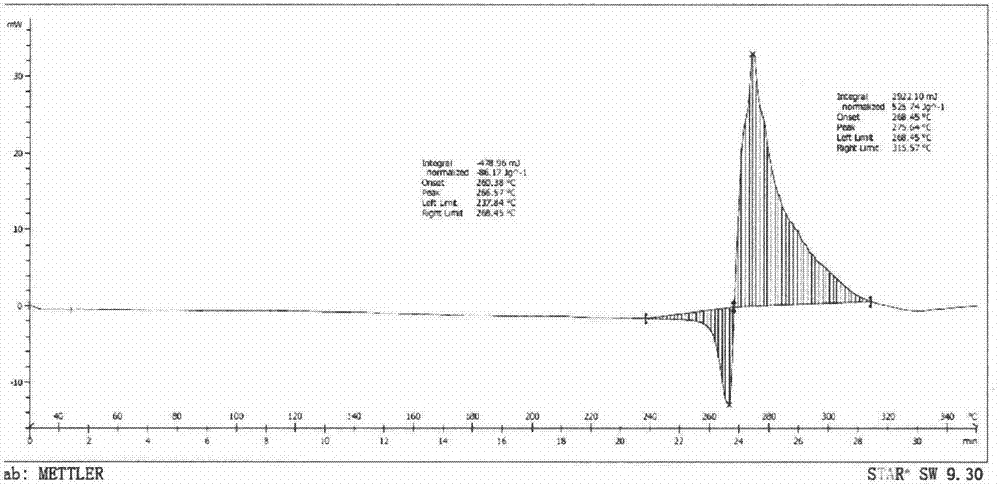
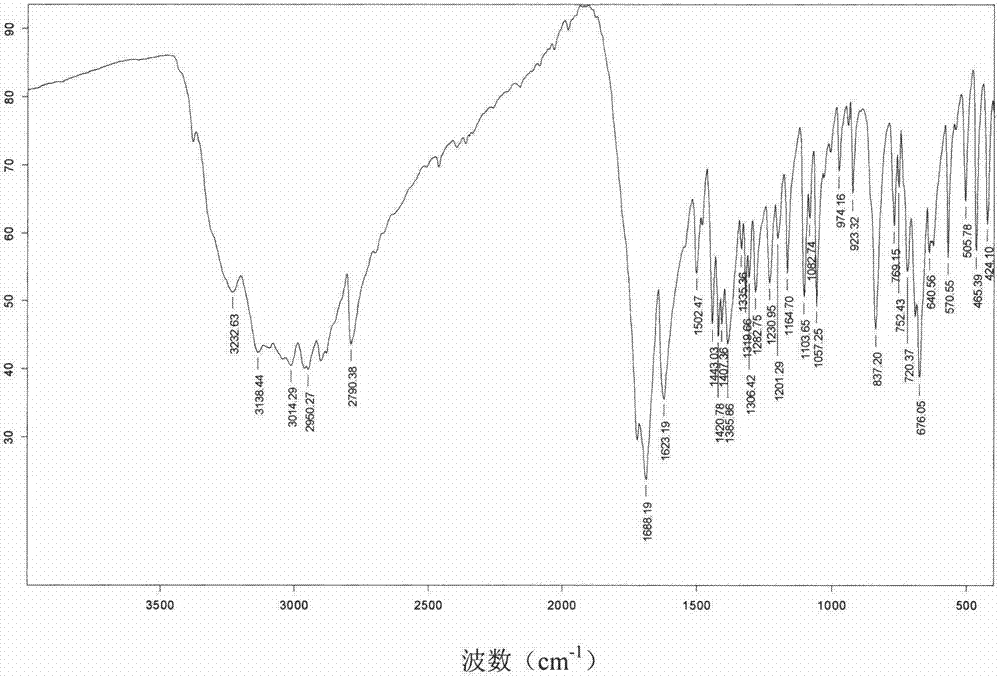
[0055] After testing, its X-ray powder diffraction pattern is as follows figure 1 As described, the differential scanning calorimetry (DSC) spectrum is as figure 2 Shown; its infrared absorption spectrum as image 3 As shown, the polarizing microscope image as Figure 4 shown.
Embodiment 2
[0056] Example 2: Preparation of 5-chloro-6-(1-(2-iminopyrrolidinyl)methyl)uracil hydrochloride crystal form I
[0057] Add 1.0 g of the solid product obtained by the method of Preparation Example 1 into a mixed solvent composed of 3.0 ml of water and 10 ml of isopropanol, heat and stir until dissolved, then cool down to 20°C within 1.5 to 2 hours while stirring, continue stirring for 2 hours, and filter. After washing and drying, 0.92 g of the crystal form I described in the title was obtained in the form of 0.92 g of white crystals, with a yield of 92% and a purity of 100.0% by HPLC;
[0058] After testing, its X-ray powder diffraction pattern is consistent with figure 1 Basically the same, differential scanning calorimetry (DSC) spectrum and figure 2 Basically the same, the infrared absorption spectrum and image 3 Basically the same, the scanning electron microscope image and Figure 4 Basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com