A method for preparing 11-aminoundecanoic acid from 10-undecenoic acid
A technology of undecanoic acid and enoic acid, which is applied in the field of preparing 11-aminoundecanoic acid from 10-undecenoic acid, can solve the problems such as non-crystallization, and achieves high quality, improved utilization rate, and improved reaction selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
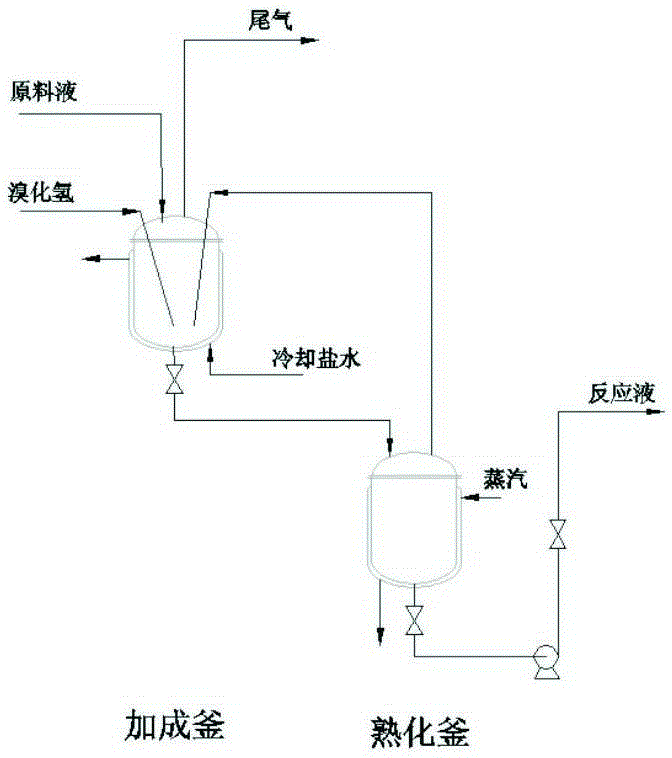
[0031] Example 1: Using 10-undecylenic acid with a melting point of 23°C and a purity greater than 98% as a raw material, the ratio of the raw material solution to 10-undecylenic acid to toluene and benzene is 1:3:2, and 10-undecylenic acid is added. 4% azobisisobutyronitrile of undecylenic acid quality, reaction device such as figure 1 , start to put about 1 / 2 volume of raw material liquid into the addition kettle first, feed hydrogen bromide gas to the bottom of the addition kettle according to the molar ratio of 10-undecylenic acid to hydrogen bromide 1:1.1, and then feed the raw material liquid with hydrogen bromide Hydrogen bromide is kept in proportion and fed continuously, cooling water or frozen brine is passed through to control the reaction temperature of the addition tank at 18-20°C, and the reaction residence time is controlled for about 45 minutes by adjusting the liquid storage in the reactor by discharging. The jacket of the aging kettle is steamed to control th...
Embodiment 2
[0034]Example 2: Using 10-undecylenic acid with a melting point of 23°C and a purity greater than 98% as a raw material, the volume ratio of the raw material liquid ratio 10-undecylenic acid to toluene and benzene is 1:3:2, and 10-undecylenic acid is added. 4% azobisisobutyronitrile of undecylenic acid quality, reaction device such as figure 1 , start to put about 1 / 2 volume of raw material liquid into the addition kettle first, feed hydrogen bromide gas to the bottom of the addition kettle according to the molar ratio of 10-undecylenic acid to hydrogen bromide 1:1.1, and then feed the raw material liquid with hydrogen bromide Hydrogen bromide is kept in proportion and fed continuously, cooling water or frozen brine is passed through to control the reaction temperature of the addition tank at 18-20°C, and the reaction residence time is controlled for about 45 minutes by adjusting the liquid storage in the reactor by discharging. The jacket of the aging kettle is steamed to con...
Embodiment 3
[0038] Example 3: Using 10-undecylenic acid with a melting point of 23°C and a purity greater than 98% as a raw material, the ratio of the raw material solution to 10-undecylenic acid and toluene, benzene The volume ratio is 1:3:2, and 10-undecylenic acid is added 4% azobisisobutyronitrile of undecylenic acid quality, reaction device such as figure 1 , start to put about 1 / 2 volume of raw material liquid into the addition kettle first, feed hydrogen bromide gas to the bottom of the addition kettle according to the molar ratio of 10-undecylenic acid to hydrogen bromide 1:1.1, and then feed the raw material liquid with hydrogen bromide Hydrogen bromide is kept in proportion and fed continuously, cooling water or frozen brine is passed through to control the reaction temperature of the addition tank at 18-20°C, and the reaction residence time is controlled for about 45 minutes by adjusting the liquid storage in the reactor by discharging. The jacket of the aging kettle is steamed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com