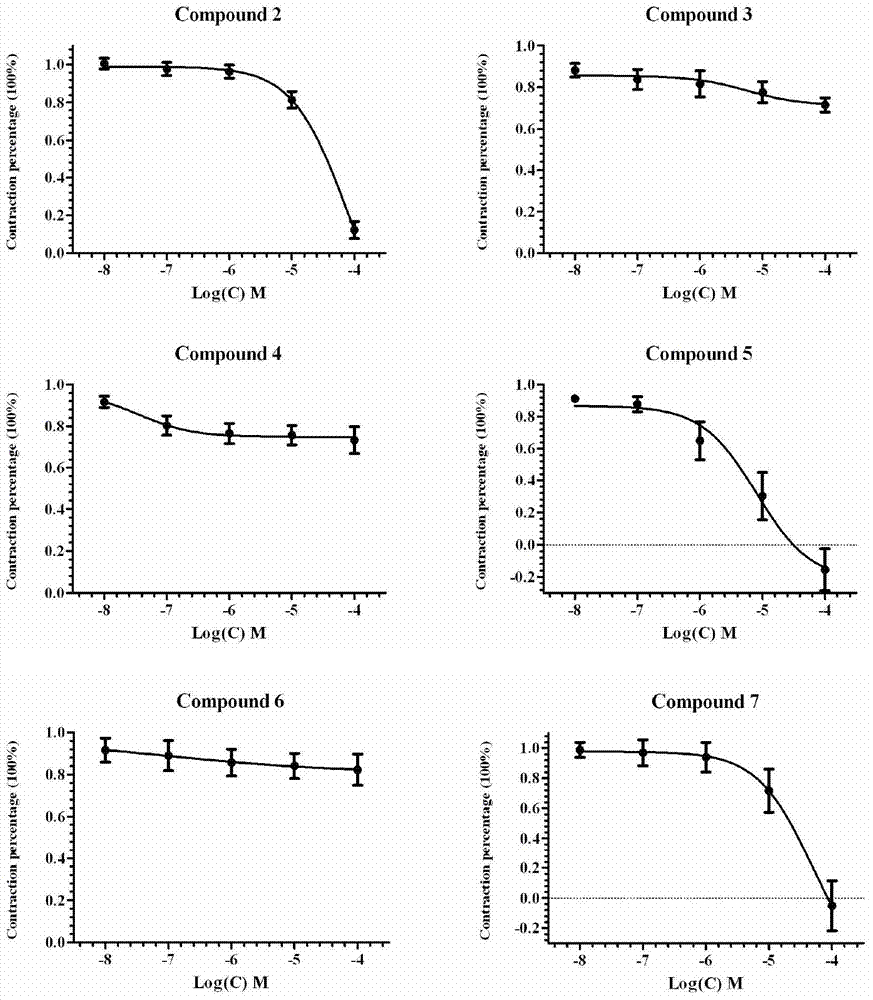
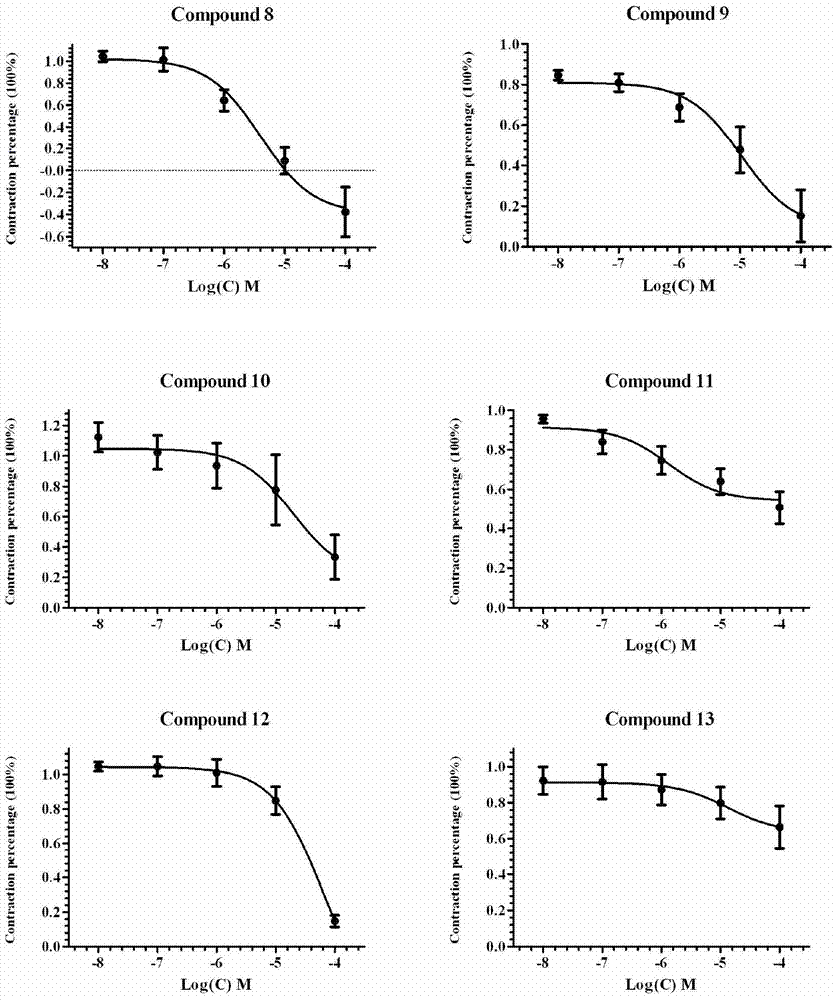
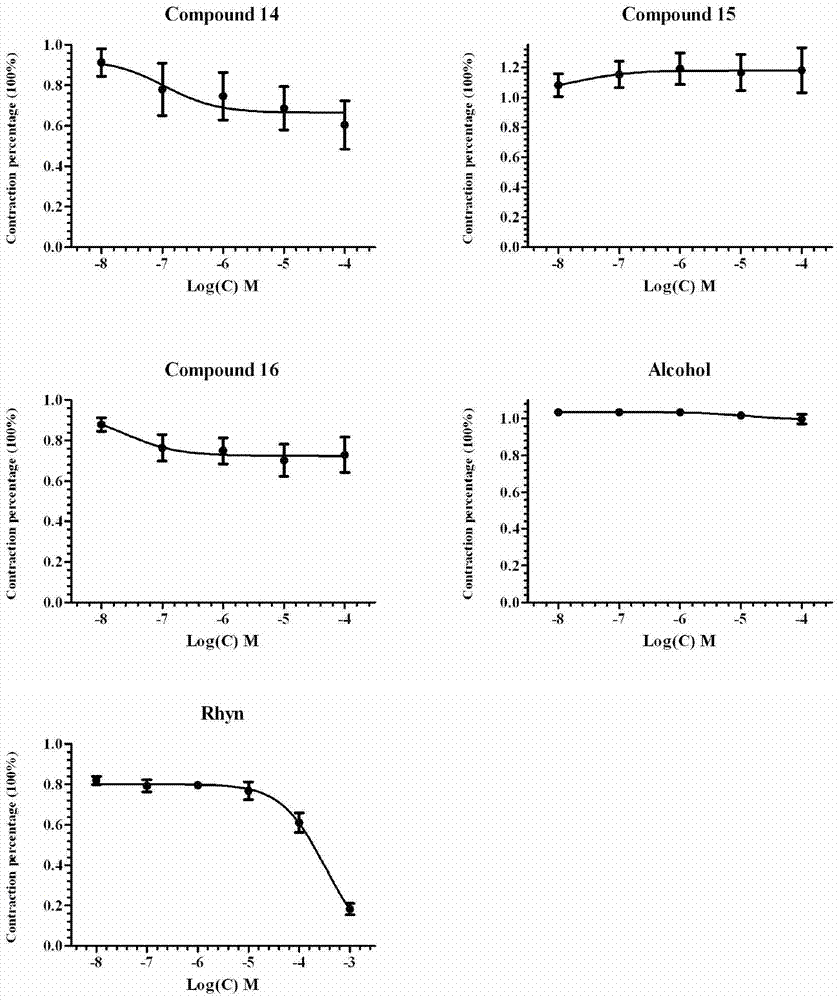
Corynantheine compound and preparation method thereof
A technology of conanne bases and analogues, applied in the field of conanne base compounds and their preparation, to achieve the effects of high yield, convenient mass production, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0038] 1: Chemical synthesis and preparation
[0039] All solvents, raw materials and reagents used in the present invention are analytically pure or chemically pure unless otherwise specified; the anhydrous treatment of solvents is carried out according to conventional methods.
[0040] Instruments and methods for product separation and identification: Thin-layer chromatography silica gel GF 254 , column chromatography coarse-pore silica gel (200-300 mesh), all produced by Qingdao Ocean Chemical Factory; TLC was detected by 154nm ultraviolet; NMR was measured by Varian VXR-500 NMR instrument, hydrogen spectrum, carbon spectrum with TMS as internal standard .
[0041] 1.1 Synthesis of 1-ethoxycarbonylmethyl-1,2,3,4-tetrahydrocarboline (Formula 2)
[0042] In a 500ml round bottom flask, add 200ml of dichloromethane dissolved with 8g (50mmol) of tryptamine and 30ml of ethyl 3,3-diethoxypropionate successively, and then slowly add 12ml of trifluoroacetic acid dropwise under sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com